5210
The potential value of PCASL in reflecting cognitive performance for older adults with MRI-negative epilepsy1Radiology Department, Huashan Hospital, Shanghai, China, 2Neurology Department, Huashan Hospital, Shanghai, China
Synopsis
Keywords: Epilepsy, Arterial spin labelling
Older adults with epilepsy accompanied by cognitive impairment represent a huge proportion, which calls for novel clinical techniques for early diagnosis and intervention. By applying pseudo-continuous arterial spin labeling (PCASL), we identified whole-brain voxel-based perfusion pattern of epilepsy associated with cognitive impairment, and explored correlations between cerebral blood flow (CBF) and domain-specific cognitive performance. The enrolled patients exhibited decreased CBF mostly in the bilateral frontal lobes, and there were positive associations between mean CBF and memory as well as executive function. Therefore, CBF measured by PCASL may be a useful indicator for cognitive performance in older adults with MRI-negative epilepsy.Introduction
Epilepsy in older adults represent a huge proportion, and its comorbidity with cognitive impairment could cause poor quality of life[1-3]. It calls for novel clinical techniques for early diagnosis and intervention in older adults with epilepsy accompanied by cognitive impairment, especially for routine MRI-negative cases. Arterial spin labeling (ASL) has advantages of noninvasiveness and quantitative measurement of cerebral blood flow (CBF), which is widely used in various cognitive disorders, including mild cognitive impairment (MCI)[4], Parkinson’s disease (PD)[5] and Alzheimer’s disease (AD)[6]. Among different labeling methods, pseudo-continuous ASL (PCASL) is recommended for its ease of implementation and high signal-to-noise ratio[7]. In this study, we identified the perfusion pattern in older adults with MRI-negative epilepsy with cognitive impairment, and investigated the correlation between CBF and domain-specific cognitive performance, aiming to explore the potential value of CBF acquired by PCASL in indicating cognitive performance in epilepsy.Methods
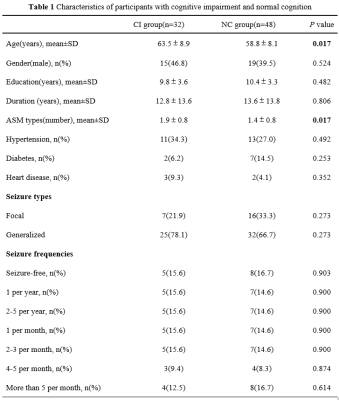
A total of 80 older adults with epilepsy diagnosis according to the International League Against Epilepsy definition[8] were recruited. They were administered a battery of neuropsychological tests[9], including (1) Mini-mental State Examination; (2) Auditory Verbal Learning Test; (3) Conflicting Instructions Task (Go/No Go Task); (4) Stick Test; (5) Modified Common Objects Sorting Test; (6) Trail-making test A&B. Z-scores were transformed from each test to evaluate five specific cognitive domains including Memory, Attention, Visuospatial function, Language and Executive function. Patients diagnosed with MCI[10] or dementia[11] were categorized into the group with cognitive impairment (CI), while the left were defined as the group with normal cognition (NC). Gender, age, years of education, duration of epilepsy, number of anti-seizure medication (ASM) types, medical history of hypertension, diabetes mellitus and heart disease, seizure types and seizure frequencies were collected. High-resolution T1 and ASL scan were conducted on a 3.0-T scanner (Discovery MR750W, General Electric, Milwaukee, USA). The CBF images were directly obtained for data processing, and whole-brain voxel-based relative CBF were compared between CI group and NC group using SPM8 software. Correlation between mean CBF of brain regions with significant differences and domain-specific cognition(z-score) was analyzed using SPSS 28.0.Results
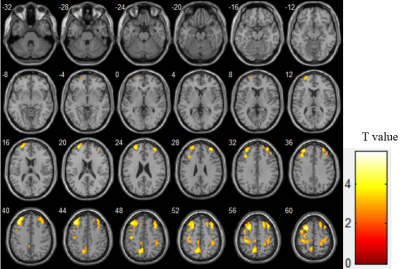
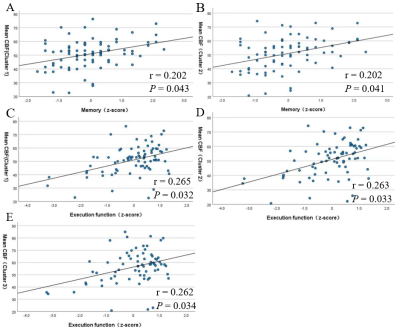
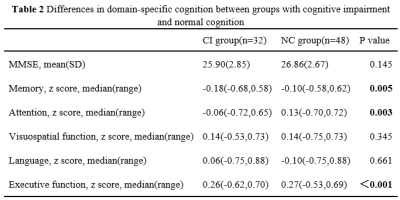
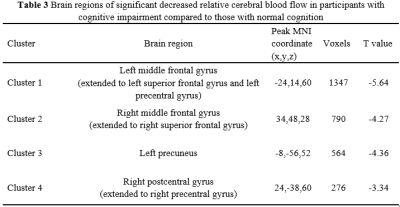
There were significant differences in age (P=0.017) and number of ASM types (P=0.017) between CI group(n=32) and NC group(n=48). Compared to NC group, CI group exhibited significantly worse performances in memory (P = 0.005), attention (P = 0.003) and executive function (P <0.001). Four clusters of hypoperfusion including the bilateral middle frontal gyrus, bilateral superior frontal gyrus, bilateral precentral gyrus, left precuneus and right postcentral gyrus were observed in CI group compared to NC group (P< 0.05, AlphaSim corrected, cluster-level). In older adults with MRI-negative epilepsy, there were positive associations between mean CBF of the bilateral middle frontal gyrus and superior frontal gyrus and memory (r = 0.202, P = 0.043 for Cluster 1 and r = 0.202, P = 0.043 for Cluster 2), and executive function (r = 0.265, P = 0.032 for Cluster 1 and r = 0.263, P = 0.033 for Cluster 2). Mean CBF of the left precuneus (Cluster 3) was also positively correlated with executive function (r = 0.262, P = 0.034).Discussion
It shows that hypoperfusion was identified in epilepsy with cognitive impairment. From the perspective of pathophysiological mechanisms, decreased CBF is thought to be a reflection of synaptic failure[12], which hinder the physiological process of a synapse transmitting information among neurons. The long-term insufficient blood oxygen and energy metabolism due to recurrent seizure attacks resulted in irreversible neuronal dysfunction, and finally impaired cognitive performance as a consequence[4]. From the perspective of clinical findings, numerous studies have found decreased CBF by ASL in healthy older individuals with deteriorated cognition[13] and patients with neurological disorders including PD-MCI[5] and AD[6]. Here, we provided further evidence on the effectiveness of CBF acquired by PCASL in discriminating impaired cognition for older adults with epilepsy. Additionally, there were positive associations between mean CBF of both the middle frontal gyrus and superior frontal gyrus and executive function respectively. The frontal lobe was found to be the most sensitive in predicting cognitive performance[14]. To be specific, the superior frontal gyrus was responsible for motor movement, working memory, resting-state and cognitive control[15], and the middle frontal gyrus was involved in attention, working memory and language-related processing[16]. Multiple researches have revealed the significant role of these two brain regions in executive function[17, 18]. Besides, as a significant part of the prefrontal cortex, the association between declined long-term memory and low metabolism of the middle frontal gyrus has been put forward in dementia[19-22]. We also noted that mean CBF of the left precuneus was positively correlated with executive function. This brain region mainly involved visuospatial and visuomotor integration, while its hypometabolism was exhibited in PD with cognitive impairment, correlating with deficient executive function[23]. The above studies accorded with the correlations between specific brain regions and relative cognitive domains in our results, which supported the potential value of CBF acquired by PCASL as a hemodynamic indicator of specific cognitive domains in older adults with epilepsy.Conclusion
Our findings suggest that CBF measured by PCASL may be a potential indicator of cognitive performance for older adults with MRI-negative epilepsy.Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (81971598, 82071456), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01) and ZJ LAB, Shanghai Academic Research Leader Program (21XD1420900), Shanghai Municipal Commission of Health (No.20224Z0002), and “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission(19SG06).
References
[1] KAESTNER E, REYES A, CHEN A, et al. Atrophy and cognitive profiles in older adults with temporal lobe epilepsy are similar to mild cognitive impairment [J]. Brain, 2021, 144(1): 236-50.
[2] KERET O, HOANG T D, XIA F, et al. Association of Late-Onset Unprovoked Seizures of Unknown Etiology With the Risk of Developing Dementia in Older Veterans [J]. JAMA Neurol, 2020, 77(6): 710-5.
[3] SEN A, JETTE N, HUSAIN M, et al. Epilepsy in older people [J]. Lancet, 2020, 395(10225): 735-48.
[4] WANG X, DING D, ZHAO Q, et al. Brain hemodynamic changes in amnestic mild cognitive impairment measured by pulsed arterial spin labeling [J]. Aging (Albany NY), 2020, 12(5): 4348-56.
[5] SHANG S, WU J, CHEN Y C, et al. Aberrant cerebral perfusion pattern in amnestic mild cognitive impairment and Parkinson's disease with mild cognitive impairment: a comparative arterial spin labeling study [J]. Quant Imaging Med Surg, 2021, 11(7): 3082-97.
[6] DUAN W, ZHOU G D, BALACHANDRASEKARAN A, et al. Cerebral Blood Flow Predicts Conversion of Mild Cognitive Impairment into Alzheimer's Disease and Cognitive Decline: An Arterial Spin Labeling Follow-up Study [J]. J Alzheimers Dis, 2021, 82(1): 293-305.
[7] ALSOP D C, DETRE J A, GOLAY X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia [J]. Magn Reson Med, 2015, 73(1): 102-16.
[8] SCHEFFER I E, BERKOVIC S, CAPOVILLA G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology [J]. Epilepsia, 2017, 58(4): 512-21.
[9] DING D, ZHAO Q, GUO Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study [J]. Alzheimers Dement, 2015, 11(3): 300-9.e2.
[10] PETERSEN R C. Mild cognitive impairment as a diagnostic entity [J]. J Intern Med, 2004, 256(3): 183-94.
[11] ASSOCIATION A P. Diagnostic and Statistical Manual of Mental Disorders, 4th edn revised [J]. Washington DC : American Psychiatric Association, 1994,
[12] LEEUWIS A E, BENEDICTUS M R, KUIJER J P A, et al. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease [J]. Alzheimers Dement, 2017, 13(5): 531-40.
[13] XEKARDAKI A, RODRIGUEZ C, MONTANDON M L, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals [J]. Radiology, 2015, 274(2): 490-9.
[14] DE VIS J B, PENG S L, CHEN X, et al. Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study [J]. J Magn Reson Imaging, 2018, 48(2): 449-58.
[15] BRIGGS R G, KHAN A B, CHAKRABORTY A R, et al. Anatomy and White Matter Connections of the Superior Frontal Gyrus [J]. Clin Anat, 2020, 33(6): 823-32.
[16] BRIGGS R G, LIN Y H, DADARIO N B, et al. Anatomy and White Matter Connections of the Middle Frontal Gyrus [J]. World Neurosurg, 2021, 150(e520-e9.
[17] SHEN Y T, YUAN Y S, WANG M, et al. Dysfunction in superior frontal gyrus associated with diphasic dyskinesia in Parkinson's disease [J]. NPJ Parkinsons Dis, 2020, 6(30.
[18] HOSMAN T, HYNES J B, SAAB J, et al. Auditory cues reveal intended movement information in middle frontal gyrus neuronal ensemble activity of a person with tetraplegia [J]. Sci Rep, 2021, 11(1): 98.
[19] WONG S, BERTOUX M, SAVAGE G, et al. Comparison of Prefrontal Atrophy and Episodic Memory Performance in Dysexecutive Alzheimer's Disease and Behavioral-Variant Frontotemporal Dementia [J]. J Alzheimers Dis, 2016, 51(3): 889-903.
[20] FUJIMOTO H, MATSUOKA T, KATO Y, et al. Brain regions associated with anosognosia for memory disturbance in Alzheimer's disease: a magnetic resonance imaging study [J]. Neuropsychiatr Dis Treat, 2017, 13(1753-9.
[21] BERTOUX M, FLANAGAN E C, HOBBS M, et al. Structural Anatomical Investigation of Long-Term Memory Deficit in Behavioral Frontotemporal Dementia [J]. J Alzheimers Dis, 2018, 62(4): 1887-900.
[22] MASSA F, GRISANTI S, BRUGNOLO A, et al. The role of anterior prefrontal cortex in prospective memory: an exploratory FDG-PET study in early Alzheimer's disease [J]. Neurobiol Aging, 2020, 96(117-27.
[23] WU L, LIU F T, GE J J, et al. Clinical characteristics of cognitive impairment in patients with Parkinson's disease and its related pattern in (18) F-FDG PET imaging [J]. Hum Brain Mapp, 2018, 39(12): 4652-62.
Figures