5204
Static and Dynamic Alteration of Intrinsic Brain Activity might improve MRI-negative Temporal Lobe Epilepsy Diagnosis and Lateralization1MRI, the first affiliated hospital of zhengzhou university, zhengzhou, China
Synopsis
Keywords: Epilepsy, fMRI (resting state), intrinsic brain activity, dynamic, cognition
We comprehensively explored the potential intrinsic brain activity (IBA) abnormalities affected by MRI-negative temporal lobe epilepsy (TLE) based on six temporal dynamic indicators (dALFF, dfALFF, dReHo, dDC, dGSCorr, dVMHC) and their corresponding static indicators, the results revealed that the abnormally activated brain regions overlap markedly, including ①decreased fALFF, Reho, DC, VMHC, dfALFF, dReHo in the temporal neocortex with ipsilateral superiority. ②decreased dGSCorr and dVMHC in the occipital lobe. Moreover, many IBA indicators significantly correlated with the epilepsy duration or cognitive scale scores. The dDC, fALFF and DC showed significant discrimination ability. The ReHo and fALFF demonstrated lateralization significance.BACKGROUND and PURPOSE
Although MRI-negative temporal lobe epilepsy (TLE) account for up to 30% of TLE cases, its intrinsic brain activity (IBA) abnormalities have been seldom investigated, especially the dynamic IBA metrics. Constantly emerging evidence has shown that mesial TLE and MRI-negative TLE probably are distinct disorders with different underlying pathophysiology and dysfunctional networks. These suggest that TLE-N should be studied separately which would promote the discovery of its specific biomarkers and epilepsy network. Therefore, in this study, we aimed to comprehensively explore the potential static and dynamic IBA abnormalities affected by MRI-negative TLE and to detect whether the changes were associated with cognition decline and epilepsy duration. Additionally, we attempted to verify whether the application of IBA indicators is conducive to the diagnosis of MRI-negative TLE and the lateralization of the epileptogenic foci.METHODS
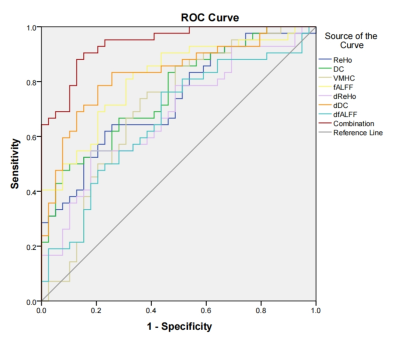
Thirty-nine unilateral MRI-negative TLE patients and 42 healthy volunteers were enrolled in the study. The resting-state functional images, 3D T1-weighted structural images, epilepsy duration years, national hospital seizure severity scale (NHS3) scores, mini-mental state examination (MMSE), memory and executive screening (MES), Montreal cognitive assessment-basic (MOCA-B), auditory verbal learning test (AVLT), shape trail making test-A/B (STT-A/B) were acquired. Using resting-state-fMRI data and DPABI software, we calculated 6 static IBA indicators (amplitude of low-frequency fluctuation (ALFF), fractional ALFF (fALFF), regional homogeneity (ReHo), and degree centrality (DC), voxel-mirrored homotopic connectivity (VMHC), global signal correlation (GSCorr)) and their 6 corresponding temporal dynamic indicators (dynamic ALFF (dALFF), dynamic fALFF (dfALFF), dynamic ReHo (dReHo), dynamic DC (dDC), dynamic VMHC (dVMHC), and dynamic GSCorr (dGSCorr)) based on a sliding window approach with a window length of 60 TR and a shifting step size of 10 TR. To examine the between-group differences, a 2-sample t test was employed with age, sex, education and mean FD as covariates. Multiple comparison correction was performed based on Gaussian random field theory (GRF, voxelwise p < 0.005, cluster-wise p < 0.05). Spearman correlation analyses were conducted between the mean IBA metrics values in regions showing group difference and the epilepsy duration, NHS3 and cognitive scores. Binary logistic regression analysis and the receiver operating characteristic (ROC) curve analysis were employed to investigate the diagnostic ability of single or combined parameters for discrimination of MRI-negative TLE and HC. For ReHo and fALFF, a pair-wise t test was employed to explore the difference between the left and right abnormally activated clusters. Cohen's d effect sizes were calculated to demonstrate the strength of the differences between the ipsilateral and contralateral sides. Moreover, we carried out additional analyses with other window sizes/step sizes (80/10 TRs) to validate our findings of the dynamic IBA indicators.RESULTS
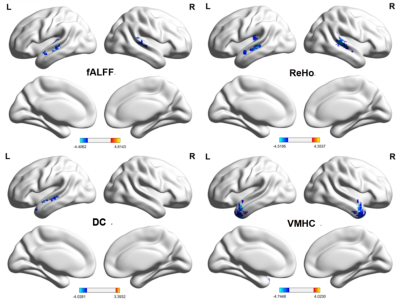
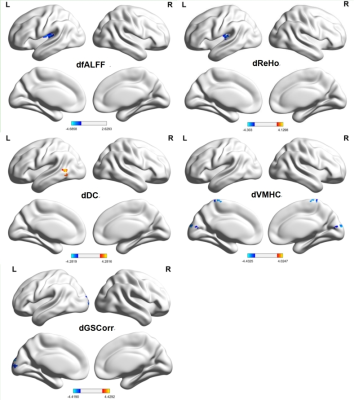
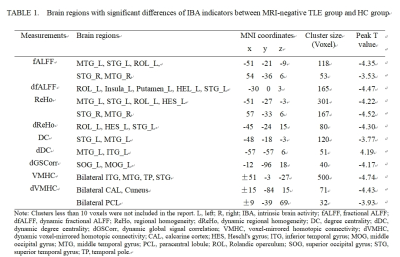
Cognitive decline was observed in MRI-negative TLE patients. MES, MOCA-B and AVLT all decreased in the TLE group (p < 0.05). However, STT-B increased in the TLE group (p < 0.05). Compared with the HC group, alterations of fALFF, dfALFF, ReHo, dReHo, DC, dDC, VMHC, dVMHC, and dGSCorr were observed in the TLE-N group (GRF corrected, Pvoxel<0.005, Pcluster<0.05). While no differences in ALFF, dALFF and GSCorr were observed after GRF correction. Marked overlap was present among the abnormal brain regions detected by different static and dynamic indicators. Firstly, altered fALFF, Reho, DC, VMHC, dfALFF, dReHo, and dDC in the temporal neocortex. including: decreased ReHo, fALFF, and VMHC values in the bilateral temporal neocortex with a larger cluster size and higher peak T value in the ipsilateral side (ReHo, fALFF); decreased DC, dReHo, dfALFF and increased dDC in the ipsilateral temporal neocortex. Secondly, decreased dGSCorr and dVMHC in the occipital lobe were observed. The correlation analysis demonstrated that many IBA indicators in the abnormal regions significantly correlated with the epilepsy duration or cognitive scale scores (P < 0.05). Furthermore, many IBA parameters (ReHo, DC, VMHC, fALFF, dReHo, dDC, dfALFF) in the temporal neocortex revealed discrimination ability of MRI-negative TLE. The dDC, fALFF and DC topped in the discrimination ability with AUC and effect sizes of 0.831/1.238, 0.812/-1.278, and 0.759/-1.053, respectively. And the largest AUC (0.940) was achieved in the combination use of the above 7 parameters. Besides, the lateralization significance of ReHo and fALFF were proved by a relatively high AUC (0.799, 0.845) and Cohen’s d (-1.234, -1.447).CONCLUSIONS
IBA abnormalities of MRI-negative TLE patients can be found in multiple brain networks related regions, including the auditory network (temporal neocortex, mainly lateralized to the epileptic side), and visual network (occipital lobe). The combination of static and dynamic IBA indices can allow conveying a more detailed and reliable description of abnormal neuronal activity and impairment or compensatory mechanisms of cognitive function, marking a step forward in the definition of a new family of biomarkers in the diagnosis and epileptogenic foci lateralization of MRI negative TLE.Acknowledgements
The authors thank all participants who participate in this study. This research study was supported by the First Affiliated Hospital of Zhengzhou University.References
Yan CG,Yang Z,Colcombe SJ,et al.Concordance among indices of intrinsic brain function:Insights from inter individual variation and temporal dynamics. Sci Bullet. 2017, 62(23):1572-1584.doi: 10.1016/j.scib.2017.09.015.
Margulies DS, Böttger J, Long X, et al. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23(5-6):289-307. doi:10.1007/s10334-010-0228-5.
Singh TB, Aisikaer A, He C, et al. The Assessment of Brain Functional Changes in the Temporal Lobe Epilepsy Patient with Cognitive Impairment by Resting-state Functional Magnetic Resonance Imaging. J Clin Imaging Sci. 2020;10:50. doi:10.25259/JCIS_55_2020.
Zeng H, Pizarro R, Nair VA, et al. Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia. 2013;54(4):658-666. doi:10.1111/epi.12066.
Shi K, Pang X, Wang Y, et al. Altered interhemispheric functional homotopy and connectivity in temporal lobe epilepsy based on fMRI and multivariate pattern analysis. Neuroradiology. 2021;63(11):1873-1882. doi:10.1007/s00234-021-02706-x.
Muhlhofer W, Tan YL, Mueller SG, et al. MRI-negative temporal lobe epilepsy-What do we know?. Epilepsia. 2017;58(5):727-742. doi:10.1111/epi.13699.
Reyes A, Thesen T, Wang X, et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484. doi:10.1111/epi.13456.
Vaughan DN, Rayner G, Tailby C, Jackson GD. MRI-negative temporal lobe epilepsy: A network disorder of neocortical connectivity. Neurology. 2016;87(18):1934-1942. doi:10.1212/WNL.0000000000003289.
Park HJ, Friston KJ, Pae C, et al. Dynamic effective connectivity in resting state fMRI. Neuroimage. 2018;180(Pt B):594-608. doi:10.1016/j.neuroimage.2017.11.033.
Chen JE, Rubinov M, Chang C. Methods and Considerations for Dynamic Analysis of Functional MR Imaging Data. Neuroimaging Clin N Am. 2017;27(4):547-560. doi:10.1016/j.nic.2017.06.009.
Li H, Ding F, Chen C, Huang P, Xu J, Chen Z, Wang S, Zhang M. Dynamic functional connectivity in modular organization of the hippocampal network marks memory phenotypes in temporal lobe epilepsy. Hum Brain Mapp. 2022;43(6):1917-1929. doi: 10.1002/hbm.25763.
Jiang S, Luo C, Huang Y, et al. Altered Static and Dynamic Spontaneous Neural Activity in Drug-Naïve and Drug-Receiving Benign Childhood Epilepsy With Centrotemporal Spikes. Front Hum Neurosci. 2020;14:361. doi:10.3389/fnhum.2020.00361.
Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339-351. doi:10.1007/s12021-016-9299-4.
Hindriks R, Adhikari MH, Murayama Y, et al. Corrigendum to "Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI?". Neuroimage. 2016;132:115. doi:10.1016/j.neuroimage.2016.02.007.
Figures