5202
The diagnostic value of mean apparent propagator (MAP) MRI in temporal lobe epilepsy (TLE) with unilateral amygdala enlargement: A pilot study1Shandong University, Jinan, China, 2Department of Radiology, Shandong Provincial Hospital, Jinan, China, 3MR scientific Marketing, Diagnostic Imaging, Siemens Healthineers Ltd, Shanghai, China, 4Department of Radiology, The Second People’s Hospital of Kunming, Kunming, China, 5The People's Hospital of Laoling, Laoling, China
Synopsis
Keywords: Epilepsy, Diffusion/other diffusion imaging techniques, mean apparent propagator (MAP)
For patients with routine MRI-negative epilepsy, noninvasive imaging is important for differential diagnosis. AE might be a subtype of MRI-negative TLE. The aim of this study was to evaluate the diagnostic efficacy of MAP-MRI and traditional DTI between TLE-AE patients and healthy controls. Results showed that MSD, QIV, RTAP, RTOP from MAP-MRI and FA from DTI in amygdala can be used as an imaging indicator to identify TLE-AE(P<0.05). Furthermore, MSD, RTAP, RTOP had a significant correlation with seizure types. MAP-MRI outperformed DTI in the diagnosis of TLE-AE, and MAP-MRI is expected to contribute in probing MRI-negative epileptogenic lesions.Introduction
Up to 30% of TLE cases show normal results on magnetic resonance imaging (MRI) results and are often labeled as “MRI-negative”, which require complex or even invasive preoperative workups to locate the lesion and have a worse surgical outcome[1]. Recently, increasing numbers of reports describe clinical and radiographic features of amygdala enlargement (AE)[2], a possible subtype of “MRI-negative” TLE[3]. There have been few reports regarding the role of amygdala and hippocampus in TLE-AE. Therefore, to avoid the complications caused by the placement of intracranial electrodes and assess the microstructure of amygdala and hippocampus, a more advanced neuro imaging method is needed. The aims of this study were as follows: (1) explore a new imaging basis for the diagnosis of MRI-negative epilepsy. (2) localization the epileptogenic foci of TLE-AE patients by DTI and MAP-MRI parameters. And compare DTI and MAP-MRI measurements to the seizure types.Methods
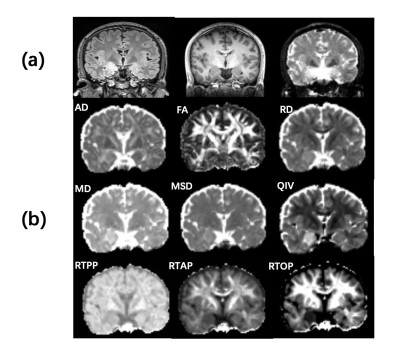
From August 2021 to September 2022, fourteen TLE-AE patients (8 women; mean age 38.43 years, range 23–66 years) and twenty-six gender-and age-matched controls (15 women; mean age 37.19 years, range 22–58 years) were prospectively recruited from our hospital. The inclusion criteria for patient group were as follows: (1) localized to one side of anterior temporal according to interictal electroencephalogram (EEG); (2) unilateral enlargement of amygdala on the ipsilateral side of the ictal onset, and conventional MRI was negative. All patients were performed using a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel head-neck coil. The MR sequences included T1w MPRAGE, T2w FLAIR and diffusion-weighted imaging (DWI). DWI was obtained by DSI sequence, and parameters was as follows: maximum b-values, 3000 s/mm2, TR, 3800 ms, TE, 72ms, FOV, 220 × 220 mm2, GRAPPA, 2, slice acceleration factor, 2, slice thickness, 2.0 mm, voxel size, 2.0 × 2.0 × 2.0 mm3, matrix size, 110 ×110, number of signal averages, 1, slices, 80, and scan time, 10.3 min. And all diffusion quantitative parameters (DTI-axial diffusivity (AD), DTI-fractional anisotropy (FA), DTI-mean diffusivity (MD), DTI-radial diffusivity (RD); MAP-mean square displacement (MSD), MAP-q-space inverse variance (QIV), MAP-return to the axis probability (RTAP), MAP-return to the origin probability (RTOP), MAP-return to the plane probability (RTPP).) were obtained by post-processing of this sequence. On MPRAGE images, the amygdala and hippocampus was manually delineated layer-by-layer using ITK-SNAP 3.8.0 to measure the volume[4]. In consideration of the difference of individual intracranial volume, we compared the laterality index [ LI= (larger side ‐ smaller side)/mean (larger side + smaller side)] of amygdala and hippocampus volume between patients and controls. Two radiologists diagnosed and sketched all patients blinded.Results
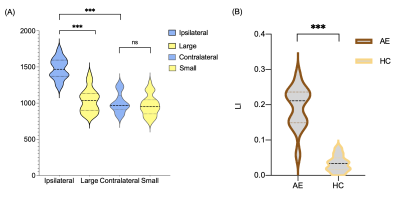
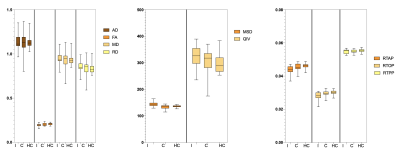
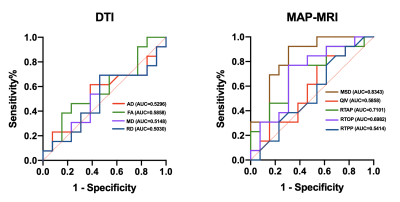
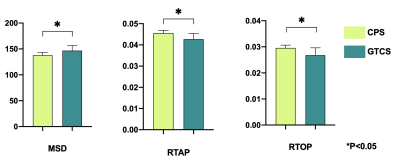
There was no significant difference in age and gender between the TLE-AE patients and the controls (P > 0.05). The amygdala volume of the affected side in patients was significantly larger than amygdala of large side in healthy controls (p < 0.001) (Fig.2). While the amygdala volume of the contralateral side in patients, was not significantly difference with amygdala of small side in healthy controls. The LI (lateral index) of patients was significantly higher than controls (p < 0.001). Fig.3 shows the box plot of DTI and MAP-MRI measurements for amygdala ipsilateral to AE, contralateral amygdala and control measurements. MSD, QIV in amygdala ipsilateral to AE were higher while FA, RTAP, RTOP were lower than these same measurements in both corresponding contralateral amygdala and controls’ amygdala (P < 0.05). However, no significant differences in MRI parameters in hippocampus were observed. The areas under the ROC curve of MAP-MRI parameters including MSD, RTAP, and RTOP in the amygdala (range, 0.69–0.83) were greater than the counterparts of QIV, RTPP of MAP-MRI and AD, FA, MD, RD of DTI (range, 0.50–0.58), which indicated that MAP-MRI had better performance in localization the epileptogenic foci of TLE-AE patients (Fig.4). The best diagnostic performance was achieved by MSD in amygdala ipsilateral to AE (AUC = 0.83). Moderate to strong correlation of seizure types with RTOP (r=-0.5916, p<0.05), MSD (r=0.6339, p<0.05) and RTAP (r=-0.6559, p <0.05) was found (Fig.5). No statistically significant correlation was found between MRI parameters and the clinical feature of TLE-AE including duration of epilepsy.Discussion
The amygdala is a small, complicated structure where numerous fibers are crossing, the assumption of a Gaussian spin displacement distribution may lead to excessive smoothing. Therefore, DTI has limitations in the characterization of regions containing multiple orientations. MAP-MRI as a novel diffusion model based on three-dimensional q-space sampling should more accurately characterize the non-Gaussian character of the three-dimensional diffusion process and features of diffusion anisotropy. Our study showed that FA from DTI and MSD, QIV, RTAP, RTOP from MAP-MRI could lateralize the side of AE. The lack of significant differences between the large and small lateral amygdala of the control group in these measurements further confirmed the epileptogenicity of AE cases.Conclusion
Our results showed that MAP-MRI metrics of amygdala with greater AUCs performed better than DTI metrics, MSD, RTAP and RTOP were associated with seizure types. These results provide further support for the validity of MAP-MRI as means to evaluate pathological structure and disease severity for TLE-AE patients.Acknowledgements
No acknowledgement found.References
[1] W. Muhlhofer, Y.L. Tan, S.G. Mueller, and R. Knowlton, MRI-negative temporal lobe epilepsy-What do we know? Epilepsia 58 (2017) 727-742.
[2] P. Singh, R. Kaur, K. Saggar, G. Singh, and S. Aggarwal, Amygdala Volumetry in Patients with Temporal Lobe Epilepsy and Normal Magnetic Resonance Imaging. Pol J Radiol 81 (2016) 212-8.
[3] Z. Fan, B. Sun, L.Q. Lang, J. Hu, N.U.F. Hameed, Z.X. Wei, Q.Y. Zhuang, J.J. Cai, F.T. Liu, Y.T. Mao, R. Feng, and L. Pan, Diagnosis and surgical treatment of non-lesional temporal lobe epilepsy with unilateral amygdala enlargement. Neurol Sci 42 (2021) 2353-2361.
[4] P.A. Yushkevich, G. Yang, and G. Gerig, ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. Annu Int Conf IEEE Eng Med Biol Soc 2016 (2016) 3342-3345.
Figures