5178
Time-dependence of perfusion fraction with flow-compensated intravoxel incoherent motion MRI in the brain1Department of Radiation Physics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Perfusion, IVIM
Using IVIM imaging for completely non-invasive perfusion MRI is gaining popularity. IVIM perfusion assessment depends not only on capillary network characteristics, but also on diffusion encoding-time. In this study, a flow-compensated pulse sequence with variable encoding-time was implemented and validated through phantom measurements, and subsequently used to study the encoding-time dependence of IVIM perfusion fraction in human brain tissue. Initial findings indicate a decrease in perfusion fraction as encoding-time increases.
Introduction
Intravoxel incoherent motion (IVIM) allows for non-invasive visualisation of molecular motions of water in tissue1. In the brain, perfusion quantification using IVIM has shown diagnostic2 and prognostic3 value for brain gliomas.To increase robustness of IVIM analysis and provide additional insight to tissue architecture, acquisition of flow-compensated (FC) and non-flow-compensated (NC) data using double diffusion-encoding (DDE) pulse sequences has been suggested4,5.
The temporal regime of IVIM depends on the capillary architecture, blood flow velocity and encoding-time. In the diffusive regime, blood flow will change direction during the encoding-time, causing pseudo-diffusion attenuation. In the ballistic regime, blood will flow in the same direction during the encoding-time, causing a phase dispersion in a capillary network which can be rephased using FC gradients, yielding a contrast between FC and NC data.
Previously, we have demonstrated encoding-time dependence of IVIM parameters in an animal tumour model using joint analysis of FC and NC data obtained on a preclinical MRI system6.
The aim of this study was to implement a single-refocused DDE pulse sequence on a clinical whole-body scanner, validate the pulse sequence through phantom measurements and use it to explore encoding-time dependence of IVIM perfusion fraction in the human brain.
Methods
Imaging protocolExperiments were performed on a 3T Philips MR 7700, using a software patch enabling arbitrary diffusion gradient waveforms7. Diffusion-weighted images were acquired using flow-compensated and non-flow-compensated DDE5, with varying encoding-time (T) (Fig. 1). T was varied by varying the duration between the end of the first bipolar diffusion gradient and the beginning of the second bipolar gradient, while keeping TE constant. Flow-compensation was enabled/disabled by reversing the polarity of the second bipolar diffusion gradient (Fig. 1)
Imaging parameters for all IVIM experiments were TE=180 ms, TR=3700 ms, six diffusion-encoding directions (sides of a cube), b=0, 5, 10, 20, 100, 200 s/mm2, encoding-time T=50, 65, 80, 100 ms, 17 slices and voxel size 2×2×4 mm3.
Phantom measurements
To validate the pulse sequence, a phantom with flowing water was constructed. The phantom consisted of a plastic pipe connected via water hoses to a water pump for continuous flow. A constant pump setting was used, generating a laminar flow profile through the transversal imaging plane. The phantom was scanned by combining the anterior and the posterior coils (together 32 channels). As a reference, a water-filled bottle was scanned simultaneously.
In vivo brain measurements
To study the encoding-time dependence of the perfusion fraction in healthy brain, a volunteer (female, 29 years) was scanned using a 32-channel head coil with the imaging protocol described above. The study was approved by the Swedish ethical review authority (ref no 2020-00029).
Preprocessing and analysis
Before analysis, the diffusion-weighted images were corrected for susceptibility and eddy-current-induced distortions using FSL tools “topup”8 and “eddy_correct”, respectively. In vivo data was segmented into white and cortical grey matter using FSL’s “FAST”9.
Assuming the ballistic regime, the IVIM signal can be represented by1,5
$$S/S_{0}=(1-f)e^{-bD}+fe^{-v_{d}^{2}c^{^{2}}/2}e^{-bD_{b}}$$where S/S0 is the normalized signal, b is the diffusion-weighting factor (s/mm2), c is the flow-weighting factor (s/mm), f is the perfusion fraction (%), vd is the velocity dispersion (mm/s), D is the tissue diffusion coefficient (mm2/s) and Db is the diffusion coefficient of blood (1.75 µm2/ms).
IVIM parameter maps for in vivo data were reconstructed by Bayesian model fitting of the ballistic signal representation, using local spatial regularization10.
Results
Phantom measurementsWith only molecular diffusion present (water bottle; Fig. 2), the signal followed an exponential diffusion attenuation (D=2.07 µm2/ms), irrespective of diffusion-encoding direction, encoding-time or flow-compensation.
Similarly, in the water flow phantom, flow-compensation or encoding-time had no effect on the signal decay when diffusion-encoding was orthogonal to the flow direction (Fig. 3a-b). As expected, the signal decreased rapidly when diffusion-encoding was aligned with the flow direction and flow-compensation was disabled (Fig. 3c). Enabling flow-compensation caused a strong rephasing of the signal.
In vivo brain measurements
As encoding-time increased, there was a general decrease in perfusion fraction in white and cortical grey matter (Fig. 4). However, in white matter f appears to reach a plateau, and in cortical grey matter f deviates from the decreasing trend at T=65 ms.
Discussion
The phantom measurements were sufficient to validate the flow-compensated pulse sequence, including independence of diffusion-encoding direction and encoding-time.For blood flow assumed to be in the ballistic regime, a sufficient increase of encoding-time implies a transition to the diffusive regime. This would explain a decrease in perfusion fraction. However, additional data is required to verify a decreasing trend.
Conclusion
An encoding-time dependence for IVIM perfusion fraction were observed in healthy human brain tissue, using flow-compensated and non-flow-compensated acquisition.Acknowledgements
The study was financed by grants from the Assar Gabrielsson Foundation, the Sahlgrenska University Hospital Research Fund, the Royal Society of Arts and Sciences in Gothenburg (KVVS), the Swedish Cancer Society, the King Gustav V Jubilee Clinic Cancer Research Foundation and Lion's Cancer Research Fund of Western Sweden, and the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement.References
1. le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168(2):497–505.
2. Federau C, Meuli R, O’Brien K, et al. Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI. American Journal of Neuroradiology 2014;35(2):256–262.
3. Federau C, Cerny M, Roux M, et al. IVIM perfusion fraction is prognostic for survival in brain glioma. Clin Neuroradiol 2017;27(4):485–492.
4. Wetscherek A, Stieltjes B, Laun FB. Flow-compensated intravoxel incoherent motion diffusion imaging. Magn Reson Med 2015;74(2):410–419.
5. Ahlgren A, Knutsson L, Wirestam R, et al. Quantification of microcirculatory parameters by joint analysis of flow-compensated and non-flow-compensated intravoxel incoherent motion (IVIM) data. NMR Biomed 2016;29(5):640–649.
6. Jalnefjord O, Rosenqvist L, Montelius M, et al. Time dependence of flow compensated intravoxel incoherent motion in tumor. In: ISMRM Annual meeting. Online; 2021. p. 2841.
7. Westin CF, Knutsson H, Pasternak O, et al. Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage 2016;135:345–362.
8. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20(2):870–888.
9. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20(1):45–57.
10. Spinner GR, Federau C, Kozerke S. Bayesian inference using hierarchical and spatial priors for intravoxel incoherent motion MR imaging in the brain: Analysis of cancer and acute stroke. Med Image Anal 2021;73.
Figures
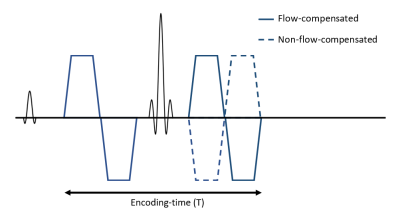
Figure 1: The pulse sequence used in this study for flow-compensated (solid) and non-flow-compensated (dashed) acquisition. To vary encoding-time (T), the duration between the end of the first and beginning of the second bipolar gradient varied, while TE was kept constant
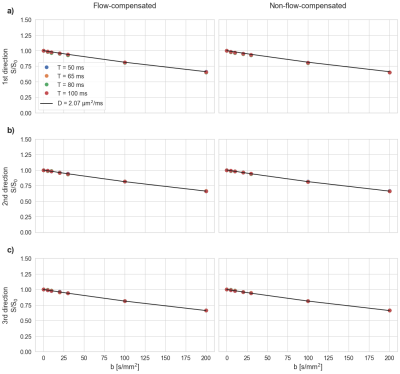

Figure 4: Mean perfusion fraction (f) for ROI in white matter (wm; left) and cortical grey matter (cgm; right), for increasing encoding time (T). As T increases, there as a general decrease in f in both wm and cgm. However, in wm f appears to reach a plateau, and in cgm f deviates at T=65 ms. A decreasing f could be explained by a temporal transition to the diffusive regime as T increases, where the phase dispersion due to perfusion can no longer be rephased using flow-compensation