5170
New biomaterial for 3D cell modeling and NMR analysis: low signal interference and high diffusion for robust metabolic studies in-vitro1Institute for Bioengineering of Catalonia, Barcelona, Spain
Synopsis
Keywords: Molecular Imaging, New Devices
Due to its non-invasive nature, NMR has become a pillar as a diagnostic technique, as well as a support for biochemical assays and disease tracking both in vivo and in-vitro. However, due to its magnetic susceptibility, some of the materials used for tissue engineering and 3D cell modeling are incompatible with the technique, interfering with data acquisition and reducing its applications in-vitro. We developed a cryogel that could help bridge this gap between NMR and tissue engineering.
Introduction
Nuclear Magnetic Resonance (NMR) is a versatile technique with the potential to be used as an insightful tool for biochemical analysis, diagnosis, and disease treatment. Last reports on applications of NMR show advances in both in-vivo and in-vitro model imaging, developing the technique further for clinical and research applications 1,2. A recent breakthrough in in-vitro disease models are 3D cell models and tissue engineering, also known as Organ-on-Chip (OoC), which recapitulate some characteristics of in vivo models 3–5. However, NMR is yet to meet OoC and these new complex bioengineered models. Certain materials used for 3D cell modeling and tissue engineering present incompatibilities with NMR detection. We propose the use of a 1% Carboxymethyl Cellulose (CMC) cryogel6 for cell modeling, long-term storage and transport, an NMR-compatible biomaterial that does not deter signal acquisition and aids in diffusion of the sample throughout the model. This cell model will open new NMR applications in-vitro with biologically complex synthetic systems.Methods
The compatibility of the cryogel with 1H and 13C NMR acquisition was assessed. The cryogel was fabricated in special cylindrical molds to occupy the entire detection region of a conventional 5 mm NMR tube and inserted into the tube containing a 0.5 M solution of [1-13C] pyruvic acid at pH 7. 1H and 13C NMR spectra were then acquired to determine the full width half maximum (FWHM) and the signal-to-noise ratio (SNR) of the NMR peaks.The suitability of the NMR-compatible biomaterial as a scaffold for 3D cell models was also investigated. Along with the spectral tests, we also verified that its diffusion and spatial integrity were optimal for cell culture. To engineer a model, AML12 (alpha mouse liver) cells were seeded onto the cryogel, where they self-aggregated and formed functional clusters. The spatial distribution and viability of these clusters was determined through confocal microscopy imaging. The viability of the 3D cell models was tested through their metabolic activity up to 11 days after assembly using the alamarBlue™ assay.
The scaffolds were further challenged on their applications by assessing their capacity to cryopreserve the models hosted inside and their viability after thawing. The viability of the cryopreserved models was analyzed after thawing and up to 11 days to assess their viability and durability. Through imaging, the CMC structures were investigated for breaks due to the cryopreservation and thawing processes. Furthermore, we tested the use of these 1% CMC cryogels to remove DMSO, the cryoprotective agent necessary when freezing biological samples, from the thawed samples due to its high cytotoxicity after thawing7 . DMSO quantification was performed using 1H-NMR analysis.
Results and Discussion
We report on an NMR-compatible biomaterial for 3D cell model engineering. This material is suitable for NMR applications, since it does not affect spectral acquisitions: the FWHM of the NMR spectra didn’t broad in 1H and broadened by 30% in 13C, and the SNR decreased by 78% (1H) and 50% (13C) when the biomaterial was present in the NMR tube (Fig. 1). The porous nature of this cryogel also presents optimal diffusion of substrates throughout the sample within seconds, a critical component that the biological models must have for some NMR experiments such as hyperpolarized NMR or perfusion studies.Due to the low availability of some samples that either come directly from clinical practice or require highly specialized equipment for their assembly, it has become imperative to construct models that can be stored and transported if necessary. We have developed a protocol that allows for cryopreservation and thawing of the cell-laden biomaterial with excellent cellular viability8. We observed there was no significant difference between the metabolic activity from the samples that underwent cryopreservation and thawing and those that remained in culture conditions (Fig. 2, D). Moreover, we determined via NMR that up to 99% of the DMSO can be removed from the sample contained in the cryogel after thawing (Fig. 3, A).
Conclusions
We have developed a biomaterial structure containing 1% CMC that can host viable 3D cell models to study biological applications by NMR. Future work will use this 3D model for metabolomics, hyperpolarization analysis and real-time biochemical assessments. Furthermore, the cryogels fabricated improve the post-cryopreservation viability of the 3D cell models, allowing for storage and transport of valuable and scarce samples to specialized centers for their analysis.Acknowledgements
AHG received financial support through the FI Fellowship Programme from AGAUR (Ref. 2021 FI_B_01039). This work is part of a project that has received funding from the Junior Leader Postdoctoral Fellowship Programme from “la Caixa” Banking Foundation (LCF/BQ/ PI18/11630020), MCIN/AEI/10.13039/501100011033 (Ref. PID2020-117859RA-I00), the European Union’s Horizon 2020 research and innovation program (GA-863037), the BIST – “la Caixa” initiative in Chemical Biology (CHEMBIO) and grant RYC2020-029099-I funded by MCIN/AEI/ 10.13039/501100011033 and by “ESF Investing in your future”.
References
1. Fan, T. W.-M. & Lane, A. N. Structure-based profiling of metabolites and isotopomers by NMR. Prog. Nucl. Mag. Res. Sp. 52, (2008).
2. Azagra, M. et al. Ammonium quantification in human plasma by proton nuclear magnetic resonance for staging of liver fibrosis in alcohol‐related liver disease and nonalcoholic fatty liver disease. NMR Biomed (2022) doi:10.1002/nbm.4745.
3. Xie, R., Zheng, W., Guan, L., Ai, Y. & Liang, Q. Engineering of Hydrogel Materials with Perfusable Microchannels for Building Vascularized Tissues. Small 16, 1902838 (2020).
4. Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat Rev Drug Discov 20, 345–361 (2021).
5. Nuciforo, S. & Heim, M. H. Organoids to model liver disease. JHEP Reports 3, 100198 (2021).
6. Velasco-Mallorquí, F., Rodríguez-Comas, J. & Ramón-Azcón, J. Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication 13, (2021).
7. Verheijen, M. et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep 9, 4641 (2019).
8. Herrero-Gómez, A., Azagra, M. & Marco-Rius, I. A cryopreservation method for bioengineered 3D cell culture models. Biomedical Materials 17, 045023 (2022).
Figures
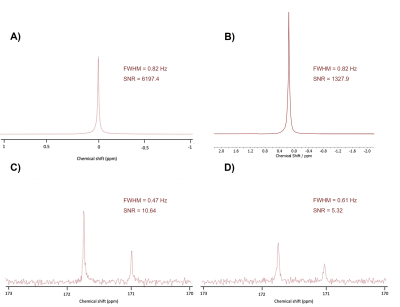
Figure 1. Compatibility of the CMC scaffold with NMR acquisition. A) 1H-NMR spectrum of a sample containing [1-13C] pyruvic acid at pH 7. B) 1H-NMR spectrum of a sample containing a cryogel in [1-13C] pyruvic acid at pH 7. C) 13C-NMR spectra of a sample containing [1-13C] pyruvic acid at pH 7. D) 13C-NMR spectra of a sample containing a cryogel in [1-13C] pyruvic acid at pH 7.
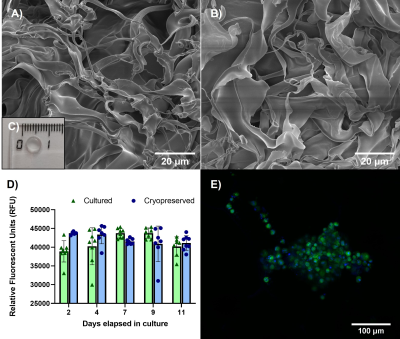
Figure 2. Characterization of the biomaterial as a suitable scaffold for cell culture. A) Scanning Electron Microscopy (SEM) image of the cryogel fibers after fabrication. B) SEM image of the fibers after cryopreservation to study structural integrity. C) CMC cryogel dimensions. D) Comparison of the metabolic activity of the AML12 cells in the cryogel at day 2,4,7,9 and 11 using the alamarBlue assay. E) Confocal image of a cluster inside the cryogel 4 days after thawing. Nuclei in blue (Hoechst 33342), apoptotic cells in red (Propidium Iodide) and cytoskeleton in green (ViaFluor 488).
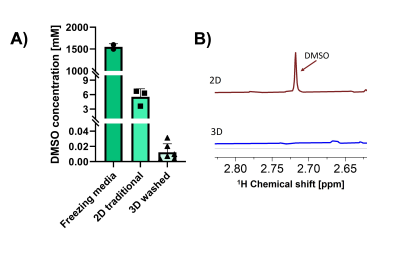
Figure 4. A) DMSO quantification via 1H-NMR in a control group (freezing media), hepatocytes frozen in suspension (2D traditional), and cell-laden scaffolds that underwent cryopreservation (3D washed). Data are presented as mean ± SD of at least two independent experiments with two replicates each. B) 1H-NMR spectrum of hepatocytes frozen in suspension (2D traditional) showing a peak identified as DMSO and 1H-NMR spectrum of a cell-laden scaffold that underwent cryopreservation without DMSO peak detected.