5168
Simultaneously Applying Multiple Diffusion Models to Predict Histologic Grade of Bladder Urothelial Carcinoma1College of Medical Imaging, Shanxi Medical University, Taiyuan, China, 2MR Scientific Marketing, Siemens Healthcare, Shanghai, China, 3Department of Radiology, The First Hospital of Shanxi Medical University, Taiyuan, China
Synopsis
Keywords: Multi-Contrast, Cancer
Tumor grading is the most important single prognostic factor for bladder urothelial carcinoma. In this study, we compared 5 diffusion models for assessing low- and high-grade in bladder urothelial carcinoma, including continuous-time random-walk (CTRW), incoherent motion within the voxel (IVIM), stretched exponential model (SEM), diffusion kurtosis imaging (DKI) and fractional-order calculus (FROC). The study found that CTRW_D, DKI_Dapp, DKI_Kapp, FROC_D, IVIM_D and SEM_DDC were significantly different between low- and high-grade bladder urothelial carcinoma and could distinguish one from the other.Introduction
Bladder cancer (BCa) is one of the most common malignancies of the genitourinary tract among man in the world1 .The majority of bladder cancer is histologically classified as urothelial carcinoma. Depending on its histopathology, it can be graded into low- and high-grade bladder tumors (LG; HG), which results in different treatment and following protocols for patients with the cancer. Transurethral resection of bladder tumor (TURBT) is a standard method to determine grading2 .However, TURBT is an inaccurate enough and an invasive examination method. Diffusion-weighted MR imaging (DWI) with apparent diffusion coefficient (ADC) that derived from Gaussian diffusion model has shown great value for grading bladder cancer3-5. Considering the complex and heterogeneous microstructure in cancer tissues, advanced non-Gaussian diffusion models may be needed to provide a more comprehensive and accurate characterization of bladder urothelial carcinoma to determine its grade6. as what has been done for hepatocellular carcinoma (HCC), uterine cervical carcinoma and gliomas7-9. To extend previous work, the aim of this study was to prospectively evaluate the performance of multiple diffusion parameters derived from non-Gaussian models for distinguishing low- from high-grade bladder urothelial carcinoma, including stretched exponential model (SEM), diffusion kurtosis imaging (DKI), intravoxel incoherent motion (IVIM) imaging, fractional order calculus (FROC) model and continuous-time random-walk (CTRW).Materials and Methods
MR imaging: A total of 60 patients with pathologically confirmed tumor lesions were included in this study from January 2022 to June 2022. All patients are divided into two subgroups (low VS High grade) according to histopathological confirmation through TURBT or cystectomy within three months after the MRI examination.Reconstruction & Segmentation: All studies were performed at a 3T MRI scanner (MAGNETOM VIDA, Siemens Healthcare, Erlangen, Germany), and the details of parameters are shown in Table 1. All above 5 DWI models: CTRW, IVIM, SEM, DKI and FROC used data from the same sequence, which were post-processed using an in-house developed software BoDiLab, which was developed using Python 3.7. Region of interest (ROI) was manually drawn on the diffusion-weighted image for each patient by two radiologists independently (with 2 and 3 years of experience in body MR diagnosis, respectively). ROI was placed as large as possible to encompass the whole tumor that was greater than 5 mm, without necrosis, cyst, hemorrhage, and submucosal stalk.
Statistical Analysis: An independent two sample t test and the Mann-Whitney U test were used to examine differences of single diffusion parameter. Receiver operating characteristic (ROC) analysis and the corresponding area under the ROC curve (AUC) were used to assess the performance of each parameter in tumor grading. The SPSS software (version 25.0) and MedCalc (version 19.6.4) were used for all the statistical analysis, with a significant level set to p values < 0.05.
Results
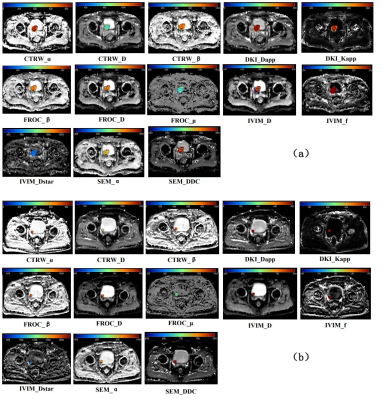
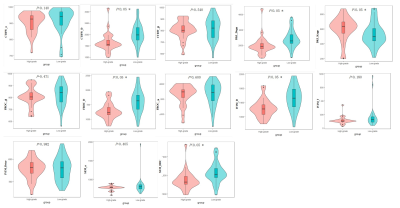
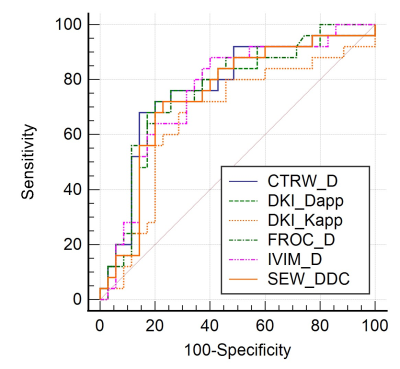
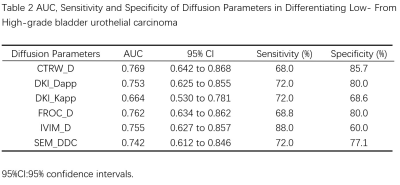
Thirty-five of 60 patients (58.33%) were confirmed by pathologic examination to have low-grade bladder urothelial carcinoma, and the remaining 25 patients (41.67%) had high-grade one.Figure 1 shows typical maps of CTRW_α, CTRW_D, CTRW_β, DKI_Dapp, DKI_Kapp, FROC_β, FROC_D, FROC_μ, IVIM_D, IVIM_f, IVIM_Dstar, SEM_α and SEM_DDC from low- and high-grade patients. Figure 2 displays the differences in the quantitative diffusion parameters between the two groups. CTRW_D, DKI_Dapp, DKI_Kapp, FROC_D, IVIM_D and SEM_DDC were significantly higher in low-grade compared to high-grade ones. Figure 3 shows the ROC curves and the corresponding AUCs of these parameters with CTRW_D having the highest AUC value of 0.769. Table 2 shows the AUC, sensitivity and specificity of diffusion parameters in differentiating low-grade from high-grade patients.
Discussion
CTRW_D, DKI_Dapp, DKI_Kapp, FROC_D, IVIM_D and SEM_DDC were significantly higher in low-grade compared to high-grade ones. This is likely attributable to higher cellularity, increased water diffusion restriction, and decreased extracellular space tortuosity in high-grade bladder cancers10.These factors contribute to the reduced motion of water molecules. CTRW_D, DKI_Dapp, DKI_Dapp and FROC_D reflects water diffusion restriction accurately at high b values, and thus can be sensitive to tissue cellularity11-14. Besides, IVIM_D indicates relatively excellent diagnostic value as well, which demonstrates that both water molecular diffusion and microcirculation perfusion correlate with the aggressive behavior of bladder cancer15. SEM_DDC can be considered the composite of individual ADCs, weighted by the volume fraction of water molecules in each part of the continuous distribution of ADCs16. Parameters from CTRW, DKI, FROC, IVIM and SEM are related but provide various types of information.Our study has limitations. First, the distribution of pathologic grades was uneven with more low-grade than high-grade tumors which may bias the statistical analysis. Second, the relatively small sample size influenced our ability to delineate low-grade than high-grade tumors.
Conclusion
Our study suggested that CTRW, IVIM, SEM, DKI and FROC models may become a potential imaging-based tool to aid histopathology for a better tumor grading for bladder urothelial carcinoma.Acknowledgements
The authors thank Xiaochun Wang and Meining Chen,whose important contributions to this study were indispensable to its success.References
1. SUNG H, FERLAY J, SIEGEL R L, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209-49.
2. WITJES J A, BRUINS H M, CATHOMAS R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021; 79(1): 82-104.
3. ZHOU G, CHEN X, ZHANG J, et al. Contrast-enhanced dynamic and diffusion-weighted MR imaging at 3.0T to assess aggressiveness of bladder cancer. Eur J Radiol. 2014; 83(11): 2013-8.
4. KOBAYASHI S, KOGA F, YOSHIDA S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011; 21(10): 2178-86.
5. TAKEUCHI M, SASAKI S, ITO M, et al. Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009; 251(1): 112-21.
6. LI Z, LI H, WANG S, et al. MR-Based Radiomics Nomogram of Cervical Cancer in Prediction of the Lymph-Vascular Space Invasion preoperatively. J Magn Reson Imaging. 2019; 49(5): 1420-6.
7. GUO Y, CHEN J, ZHANG Y, et al. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models. Eur J Radiol. 2022; 150: 110237.
8. LIN M, YU X, CHEN Y, et al. Contribution of mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging in the diagnosis and differentiation of uterine cervical carcinoma. Eur Radiol. 2017; 27(6): 2400-10.
9. GAO A, ZHANG H, YAN X, et al. Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology. 2022; 302(3): 652-61.
10. PADHANI A R, LIU G, KOH D M, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia (New York, NY). 2009; 11(2): 102-25.
11. TANG L, ZHOU X J. Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging. 2019; 49(1): 23-40.
12. KARAMAN M M, ZHANG J, XIE K L, et al. Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR in biomedicine. 2021; 34(4): e4485.
13. LI Q, CAO B, TAN Q, et al. Prediction of muscle invasion of bladder cancer: A comparison between DKI and conventional DWI. Eur J Radiol. 2021; 136: 109522.
14. FENG C, WANG Y, DAN G, et al. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur Radiol. 2022; 32(2): 890-900.
15. ZHANG M, CHEN Y, CONG X, et al. Utility of intravoxel incoherent motion MRI derived parameters for prediction of aggressiveness in urothelial bladder carcinoma. Journal of Magnetic Resonance Imaging. 2018; 48(6): 1648-56.
16. BAI Y, LIN Y, TIAN J, et al. Grading of Gliomas by Using Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MR Imaging and Diffusion Kurtosis MR Imaging. Radiology. 2016; 278(2): 496-504.
Figures