5157
Quantifying inhomogeneity of kidney, liver and muscle before and after the freeze-thaw cycle using 1-Norm waveform analysis and MR elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2BIH Biomedical Innovation Academy, BIH Charité Digital Clinician Scientist Program, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany, 3Radiology, Mayo Clinic, Rochester, MN, United States, 4Richard and Loan Hill Department of Biomedical Engineering, University of Illinois Chicago, Chicago, IL, United States
Synopsis
Keywords: Elastography, Tissue Characterization
The recently developed 1-Norm technique can be used to quantify the extend of scattering of mechanical waves generated by inhomogeneities. This explorative study shows that 1-Norm can detect mechanical inhomogeneity of ex vivo kidney, liver and muscle without the need to use ill-posed wave inversion techniques. The 1-Norm technique has potential to serve as an MRE-based diagnostic biomarker independent of stiffness for the characterization of pathological conditions associated with changes in tissue mechanical inhomogeneity.
Introduction
In spite of the success of MR elastography (MRE) in assessing biophysical tissue properties, current MRE methods use ill-posed wave inversions to estimate tissue stiffness. The recently developed 1-Norm technique can be used to quantify the extend of scattering of mechanical waves generated by inhomogeneities such as cross-links in a 3D printed fiber phantom or fibers in muscle tissue.1 1-Norm is a dimensionless biomarker and a measure for the derivation of mechanical shear wave fronts from idealized geometries, such as planes and spheres.1 Increased values of 1-Norm indicate increased wave scattering and an increased degree of inhomogeneity.Freezing is a technique for preserving biological specimens.2 In comparison to conventional fixation in formalin, frozen tissue banks may provide access to large quantities of well-preserved specimens for testing with low-cost tabletop MRE. Although there is recent evidence that the freeze-thaw cycle (FTC) decreases stiffness in kidney, liver and muscle tissue, little is known about the effects of the FTC on biophysical properties due to microscopic structural tissue changes.3 Therefore, we explored 1-Norm as an MRE-based quantitative biomarker of mechanical inhomogeneity compared to conventional wave inversion methods. We hypothesize that the FTC yields detectable alterations in the mechanical inhomogeneity of biological tissues.
Methods
In this explorative study, we prospectively examined fresh ex vivo porcine kidney (n=6), liver (n=6) and muscle (n=6) before and after the FTC at 500, 1000, 1500 and 2000 Hz with 0.5T tabletop MRE (Pure Devices, Würzburg, Germany) as described in 4,5. Samples were scanned before the FTC and then frozen at -20˚C for 24 hours, fully thawed to room temperature (23˚C, approx. 2 h), and the same set of MRE experiments was repeated. Scan parameters were: field of view, 10mm×10mm; matrix, 128×128; number of slices, 1; slice thickness, 5 mm; TE, 35.8ms; TR, 500ms; number of averages, 1; number of time steps, 4; pulse sequence, spin echo. 1-Norm as a representation of the degree of inhomogeneity was compared with the conventional wave inversion, where stiffness was represented by the real part of the complex shear modulus. 1-Norm data processing is described in detail in 1. Of note, this study is complementary to previously published data on the rheological modeling of porcine kidney, liver and muscle tissue.3 The reason for this complementary analysis was that the previously used methods were constrained in assessing mechanical inhomogeneity.Results
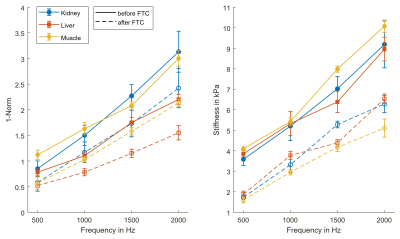
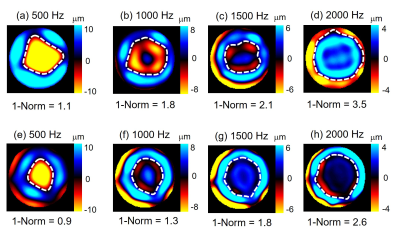
Group values of kidney, liver and muscle over frequency before and after the FTC are illustrated in figure 1, and a representative case of kidney tissue is shown in figure 2. A significant decrease was found for all tissues and frequencies for both 1-Norm and stiffness after the FTC (P<0.05), except for kidney tissue at 500 Hz (P=0.052) and frequency-averaged values (P=0.11) where a non-significant trend for decrease was found. Group and frequency-averaged values showed an FTC-related decreased of both 1-Norm and stiffness as follows: kidney by -23.7% and -33.6% (1.94±0.85 vs. 1.48±0.68, p=0.110; and 6.25±2.09 kPa vs. 4.15±1.76 kPa, p=0.003), liver by -31.5% and -32.5% (1.46±0.55 vs. 1.00±0.39, P=0.006; and 6.13±1.86 kPa vs. 4.14±1.66 kPa, P=0.003), and muscle by -32.1% and -49.9% (1.96±0.69 vs. 1.33±0.59, P=0.007; and 6.89±2.30 kPa and 3.45±1.31 kPa, P<0.001).Discussion
In this exploratory study, we investigated 1-norm as a quantitative biomarker of tissue mechanical inhomogeneity compared to conventional wave inversion techniques, which represent ill-posed problems. This study shows that the FTC resulted in measurable changes in tissue mechanical inhomogeneity. Our results show an FTC-related decrease of both inhomogeneity and stiffness in the order of approximately -30% and -30% for ex vivo kidney and liver, and by -30% and -50% for muscle, respectively. Additionally, an increased inhomogeneity was found in muscle compared with kidney and liver, which may be explained by the presence of muscle fibers, which have anisotropic properties compared with the isotropic stroma of kidney and liver. Decreased inhomogeneity and stiffness after the FTC might be related to the degradation of extracellular matrix components, damaged cell wall integrity, and cell wall detachment.6The effects of inhomogeneities on shear wave propagation have been investigated previously by Papazoglou et al.7 The authors found that different regimens of shear wave diffusion were depending on experimental parameters including shear wave length, mean scatterer spacing and stiffness of the scatterers. Nevertheless, in the present study, a different approach was taken by analyzing the wave front distortion as a mathematical parameter. Our explorative study may pave the way to use 1-Norm as a biomarker for diseases that lead to spatially inhomogeneous tissue properties, such as cancer, chronic liver diseases8 and Alzheimer’s disease9. Further studies with histopathological reference are needed to elucidate the microscale causes of the changes in mechanical properties in frozen-tissue bank samples subjected to an FTC. Our study has limitations. First, we only investigated a small number of specimens in this explorative study. Second, there was no histopathological assessment of porcine specimens.
Conclusion
This explorative study shows that 1-Norm can detect mechanical inhomogeneity of biological tissues without the need to use ill-posed wave inversion techniques. The 1-Norm technique has the potential to serve as an MRE-based diagnostic biomarker independent of stiffness for the characterization of pathological conditions associated with changes in tissue mechanical inhomogeneity.Acknowledgements
This work was supported by the NSF Grant CMMI-1935462, NSF Grant No. 1852691, NIH Grant No. AR071162 and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, RE 4161/1-1, RE 4161/1-2, RE 4161/2-1, Rolf Reiter). Rolf Reiter is a participant of the BIH-Charité Digital Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, the Berlin Institute of Health and the DFG.
References
1. Palnitkar H, Reiter RO, Majumdar S, Lewis P, Hammersley M, Shah RN, et al. An investigation into the relationship between inhomogeneity and wave shapes in phantoms and ex vivo skeletal muscle using Magnetic Resonance Elastography and finite element analysis. J Mech Behav Biomed Mater. 2019;98:108-20.
2. Shabihkhani M, Lucey GM, Wei B, Mareninov S, Lou JJ, Vinters HV, et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem. 2014;47(4-5):258-66.
3. Reiter R, Zampini MA, Guidetti M, Majumdar S, Royston TJ, Klatt D. Tabletop MR elastography for investigating effects of the freeze-thaw cycle on the mechanical properties of biological tissues. J Mech Behav Biomed Mater. 2022;135:105458.
4. Guidetti M, Zampini MA, Jiang Y, Gambacorta C, Smejkal JP, Crutison J, et al. Axially- and torsionally-polarized radially converging shear wave MRE in an anisotropic phantom made via Embedded Direct Ink Writing. J Mech Behav Biomed Mater. 2021;119:104483.
5. Zampini MA, Guidetti M, Royston TJ, Klatt D. Measuring viscoelastic parameters in Magnetic Resonance Elastography: a comparison at high and low magnetic field intensity. J Mech Behav Biomed Mater. 2021;120:104587.
6. de Schellenberger AA, Tzschatzsch H, Polchlopek B, Bertalan G, Schrank F, Garczynska K, et al. Sensitivity of multifrequency magnetic resonance elastography and diffusion-weighted imaging to cellular and stromal integrity of liver tissue. J Biomech. 2019;88:201-8.
7. Papazoglou S BJ, Klatt D, Sack I. Shear Wave Diffusion Observed by Magnetic Resonance Elastography. New Developments in the Visualization and Processing of Tensor Fields. 2012:157–68.
8. Reiter R, Shahryari M, Tzschatzsch H, Klatt D, Siegmund B, Hamm B, et al. Spatial heterogeneity of hepatic fibrosis in primary sclerosing cholangitis vs. viral hepatitis assessed by MR elastography. Scientific Reports. 2021;11(1):9820.
9. Majumdar S, Klatt D. Longitudinal study of sub-regional cerebral viscoelastic properties of 5XFAD Alzheimer's disease mice using multifrequency MR elastography. Magn Reson Med. 2021;86(1):405-14.
Figures