5155
Mesenteric Adipose Tissue in Crohn’s Disease assessed by in vivo MR Elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Surgery, Charité - Universitätsmedizin Berlin, Berlin, Germany, 3Surgery, Parkklinik Weißensee, Berlin, Germany, 4Gastroenterology, Infectious Diseases and Rheumatology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 5iPATH.Berlin-Immunopathology for Experimental Models, Charité - Universitätsmedizin Berlin, Berlin, Germany, 6Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Keywords: Elastography, Body, inflammatory bowel disease
Despite increasing evidence that the functional involvement and structural changes of mesenteric adipose tissue influence the course of Crohn's disease, its viscoelastic properties remain elusive. We demonstrate the feasibility of in vivo MR elastography of mesenteric adipose tissue and present preliminary reference values for Crohn's disease patients and healthy controls in this explorative study. Our preliminary results show an excellent diagnostic performance in detecting Crohn's disease by assessing the viscoelastic properties of mesenteric adipose tissue using histopathology of surgical specimens as reference. Our results motivate further studies for the biophysical characterization of mesenteric adipose tissue in inflammatory bowel disease.
Introduction
Despite increasing evidence that the functional involvement and structural changes of mesenteric adipose tissue (MAT) influence the course of Crohn's disease (CD), its viscoelastic properties remain elusive. CD is a severe disorder characterized by chronic recurrent inflammation that can lead to intestinal and extraintestinal complications such as strictures and abscess formation.1 Although the underlying pathology remains unclear, an increased permeability of the intestinal wall with translocation of bacteria to the adjacent MAT is thought to play an active role in disease progression.2 Recently, there has been increasing evidence that structural changes in MAT –so called creeping fat– influence the progression, prognosis, and outcome of CD.3,4 We hypothesize that the development of creeping fat in response to CD-related inflammation results in altered tissue properties. Therefore, we investigated the viscoelastic properties of MAT in CD patients and healthy volunteers using in vivo MR elastography (MRE).Methods
The study was approved by the local IRB and written informed consent was obtained from all subjects. This explorative study was a secondary analysis of prospectively collected data at a single institution, and MRE data of the bowel wall have been reported previously.5 Eleven CD patients with clinical indication for surgery and 20 healthy controls were examined on a 1.5T MRI scanner (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) using multifrequency MRE at 40-70 Hz and tomoelastography postprocessing6-8, yielding shear wave speed (SWS, in m/s) and loss angle of the complex modulus (φ in rad) representing stiffness and viscosity-related dispersion of stiffness over frequency, respectively.7,9,10 No oral or intravenous contrast media or spasmolytic agents were administered. Surgery was performed the day after the MRE scan. Volumes of interest were drawn in the MAT adjacent to CD lesions (MATCD) and on the opposite side without lesions in CD patients (MATCD_Opp) and healthy controls (MATCTRL).Results
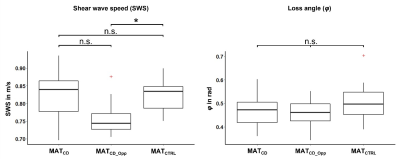
Mean age and mean body mass index was 41 ± 15 years and 21.8 ± 3.4 kg/m2 for patients (n = 11, 6 women), and 37 ± 14 years and 22.0 ± 2.6 kg/m2 for healthy controls (n = 20, 10 women). A case of a CD patient is shown in figure 1. As shown in figure 2, there was a statistically significant 7% decrease in mean SWS for MATCD_Opp compared to MATCTRL (0.76 ± 0.05 m/s vs. 0.82 ± 0.04 m/s, p = 0.012), while there was a non-significant trend with an 8% increase for MATCD compared to MATCD_Opp (0.82 ± 0.07 m/s vs. 0.76 ± 0.05 m/s, p = 0.098) and no difference for MATCD compared to MATCTRL. Preliminary area under the receiver operating characteristic (AUROC) analysis showed that diagnostic performance in detecting CD was excellent for SWS of MATCD_Opp (AUROC = 0.82) but poor for SWS of MATCD (AUROC = 0.52).Discussion
We demonstrate the feasibility of in vivo MRE of MAT and present preliminary reference values for CD patients and healthy controls in this explorative study. AUROC results show that the diagnostic performance in detecting CD was excellent for SWS of MATCD_Opp (AUROC = 0.82) but poor for SWS of MATCD (AUROC = 0.52). A statistically significant 7% decrease in mean stiffness was found for MATCD_Opp compared to MATCTRL. It is possible that this indicates a general softening of MAT in CD, with a (statistically non-significant) trend toward 8% higher stiffness in areas of focal inflammatory bowel lesions (MATCD). This result may be related to increased density of network elements by particularly small adipocytes in CD and increased leukocyte infiltration, which contribute to the secondary barrier function of creeping fat. Moreover, this may represent the biophysical signature that prevents the translocation of bacteria from intestinal walls with CD-related higher permeability, thus reducing the inflammatory response from systemic to localized. Nevertheless, further studies are needed to support this preliminary hypothesis. To date, few imaging studies of MAT in CD have been performed, and even fewer have used quantitative MRI. For instance, van Schelt et al. studied patients with active CD (n = 7) and healthy subjects (n = 7) with tomoelastography of 30-60 Hz and histopathologic assessment in a subset of the patients.11 The authors found no significant difference for mean SWS (CD: 0.80 ± 0.21 m/s vs. healthy: 0.67 ± 0.07 m/s, p = 0.18), whereas a significant increase was found for mean φ (CD: 0.58 ± 0.15 rad vs. healthy: 0.45 ± 0.08 rad, p = 0.02). While our results are in the same order of magnitude, we found no significant group differences for φ. This may be related to the use of mannitol solution as an oral contrast agent in patients and to the increased inflammation in active CD compared with our study with more fibrotic CD. Our study has limitations. We studied only a small number of subjects in this exploratory study. Moreover, no spasmolytic agents and no oral contrast agents were administered to allow comparison with healthy controls, yet intestinal adhesions and pathologically altered movements in CD may have influenced the comparison with healthy controls.Conclusion
We demonstrate the feasibility of in vivo MRE of MAT and present preliminary reference values for CD patients and healthy controls in this explorative study.Acknowledgements
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): SFB 1340/1 “Matrix in Vision” project number 372486779 (Bernd Hamm, Jürgen Braun, Ingolf Sack, Patrick Asbach, Anja A. Kühl and Britta Siegmund), GRK 2260 BIOQIC (Ingolf Sack, Jürgen Braun), RE 4161/2-1 (Rolf Reiter), project number 467843609 (Stephan R Marticorena Garcia), and SFB-TR 241 “IEC in IBD” (Anja A. Kühl, Carl Weidinger and Britta Siegmund). Rolf Reiter is a participant of the BIH-Charité Digital Clinician Scientist Program funded by Charité – Universitätsmedizin Berlin, Berlin Institute of Health and the DFG.
References
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741-55.
2. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516-26.
3. Siegmund B. Mesenteric fat in Crohn’s disease: the hot spot of inflammation? Gut. 2012;61(1):3-5.
4. Kredel L, Batra A, Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. 2014;30(6):559-65.
5. Reiter R, Loch FN, Kamphues C, Bayerl C, Marticorena Garcia SR, Siegmund B, et al. Feasibility of Intestinal MR Elastography in Inflammatory Bowel Disease. J Magn Reson Imaging. 2022;55(3):815-22.
6. Dittmann F, Hirsch S, Tzschatzsch H, Guo J, Braun J, Sack I. In vivo wideband multifrequency MR elastography of the human brain and liver. Magn Reson Med. 2016;76(4):1116-26.
7. Tzschatzsch H, Guo J, Dittmann F, Hirsch S, Barnhill E, Johrens K, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1-10.
8. Marticorena Garcia SR, Hamm B, Sack I. Tomoelastography for non-invasive detection and treatment monitoring in acute appendicitis. BMJ Case Rep. 2019;12(8):e230791.
9. Streitberger K-J, Lilaj L, Schrank F, Braun J, Hoffmann K-T, Reiss-Zimmermann M, et al. How tissue fluidity influences brain tumor progression. Proceedings of the National Academy of Sciences. 2020;117(1):128-34.
10. Guo J, Bertalan G, Meierhofer D, Klein C, Schreyer S, Steiner B, et al. Brain maturation is associated with increasing tissue stiffness and decreasing tissue fluidity. Acta Biomaterialia. 2019;99:433-42.
11. van Schelt A, Beek K, Wassenaar NPM, Schrauben EM, Nederveen AJ, Stoker J. MR Elastography of the Affected Mesenteric Fat in Active Crohn’s Disease: A Feasibility Study. Proc Intl Soc Mag Reson Med. 2022;16.
Figures
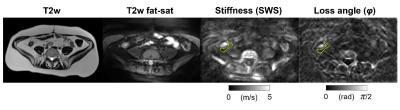
Figure 1. 41-year-old woman with Crohn’s disease of the terminal ileum with shear wave speed (SWS) of 0.80 ± 0.10 m/s and loss angle (φ) of 0.47 ± 0.05 rad in the mesenteric adipose tissue around the terminal ileum. The region of interest is indicated by the yellow dashed line.