5151
Response of Brain Tissue Mechanical Properties to Applied Pre-Strain in Magnetic Resonance Elastography1Biomedical Engineering, University of Delaware, Newark, DE, United States
Synopsis
Keywords: Elastography, Traumatic brain injury
This study uses magnetic resonance elastography (MRE) with incremental compression to measure the apparent viscoelastic properties of bovine brain tissue as pre-strain is applied. Results from our tissue-agar phantom showed an increase in stiffness, and a decrease in damping ratio for the bovine brain. The agar showed an increase in stiffness and no consistent change in damping ratio. This research serves as the foundation for future studies measuring the nonlinear properties of human cadaveric brain tissue. Output from this work will be instrumental in advancing current traumatic brain injury (TBI) models.
Introduction
Traumatic brain injury (TBI) is a neuropathology caused by blunt or blast impact to the skull and brain, which affects approximately 70 million people per year1. Despite extensive research, much is still unknown about the mechanisms of TBI. Computational models are often used to study TBI, however they require physiologically relevant mechanical properties as input parameters. We can determine these mechanical properties, such as stiffness and damping ratio, using magnetic resonance elastography (MRE)2. Tissue damage from TBI involves significant strain outside of its elastic range, which causes permanent damage and disruption of typical brain function3. Traditional in vivo MRE, however, is limited to characterization of linear viscoelastic properties of brain tissue4 with induced strains on the order of approximately 10-4 to 10-3. Experiments analyzing mechanical properties with greater strains and strain rates are required for accurately modeling injury events associated with TBI, though they are unable to be safely performed in vivo. Here we implement a method for performing MRE with increased levels of applied compressive pre-strain in a phantom system with ex vivo brain tissue to develop a better understanding of viscoelastic properties for TBI modeling.Methods
Samples: Fresh calf bovine brain samples were purchased through a local butcher and stored at 4°C for four days. Prior to sectioning, samples were then thawed for three hours. We cut the brain tissue to approximately 14 x 9 x 4 cm3 sections using a scalpel. These samples were then encapsulated in a 0.65 wt.% agarose phantom and placed at 4°C overnight to gel. The tissue-agar phantoms were brought to room temperature prior to scanning. Agar was used as an encapsulation material to assist with wave penetration and minimize boundary conditions during mechanical property estimates.Imaging: MRE data was collected on two tissue-agar phantoms using a Siemens 3T Prisma MRI scanner with a 20-channel head coil. We used an echo-planar imaging (EPI) sequence with the following imaging parameters: 240 x 240 mm field-of-view, 2.5 mm3 isotropic resolution, 60 slices, TE/TR = 67/7214 ms. The Resoundant pneumatic actuation system (Rochester, MN) was used to induce 75 Hz vibrations and 4 phase offsets were sampled.
Pre-Strain: To apply pre-strain, the tissue-agar phantom was axially compressed using a manual compression device. MRE images were collected at baseline (uncompressed) and two levels of compression (approximately 10% and 20% strain). The amount of applied compressive pre-strain achieved was determined during post-processing using MRE magnitude images to calculate the change in height of the tissue sample relative to the total precompression height. Figure 1 illustrates a representative slice from one phantom at the baseline and moderate compression levels.
Analysis: A nonlinear inversion algorithm (NLI) was used to determine the mechanical properties of the tissue and agar components simultaneously5. Viscoelastic property maps for stiffness and damping ratio were estimated for each compression level6. Regions of interest (tissue and agar) were then segmented from the phantom using MRE magnitude images, and values for stiffness and damping ratio were averaged in each region. For the bovine brain, 10 slices with the full tissue sample present were selected for analysis. For analyzing the surrounding agar, 8 sequential slices with minimal brain tissue present were selected.
Results
Beyond baseline, we achieved two pre-strain levels for each phantom. The applied pre-strain resulted in light compression levels of 11% and 9.1% and moderate compression levels of 22% and 18.2% for each sample, respectively. As the phantoms were compressed the apparent stiffness of the brain tissue increased by 23.0% and 31.0% respectively (Figure 2). Conversely, as applied pre-strain increased, the apparent damping ratio of the tissue decreased by 41.0% and 29.1% (Figure 3). We also saw the agar stiffness increase by 58.2% and 99.6%. This increase in stiffness is comparable to that in brain tissue, though the agar had a softer initial baseline. The apparent damping ratio of the agar was inconsistent without clear increase or decrease as compression was applied. Stiffness and damping ratio values for both samples, at each compression level, are displayed in the table shown in Figure 4.Discussion & Conclusion
MRE was used to observe the relationship between applied pre-strain and viscoelastic properties through incremental compression. Our preliminary results show that as compressive pre-strain is applied the stiffness of brain tissue increases and the damping ratio decreases, which supports our initial hypothesis. Although this study served as proof-of-concept for the experimental protocol, in subsequent investigations we will ensure to follow mechanical testing guidelines for precise sample preparation. To standardize tissue preparation, we will laser cut our brain tissue samples ensuring precise and consistent dimensioning of samples. Future work seeks to use MRE with pre-strain to characterize the nonlinear viscoelastic properties of bovine and human cadaveric brain tissue at expected TBI-inducing strain levels. Long term applications of this study will use nonlinear property values to supplement current computational TBI models.Acknowledgements
This work was supported by the NIH grant U01-NS112120 and an NIH Bench-to-Bedside grant.
References
1. Wiles, M. D. Management of traumatic brain injury: a narrative review of current evidence. Anaesthesia 77, 102–112 (2022).
2. Bayly, P. V. et al. MR Imaging of Human Brain Mechanics In Vivo: New Measurements to Facilitate the Development of Computational Models of Brain Injury. Ann. Biomed. Eng. 49, 2677–2692 (2021).
3. Boulet, T., Kelso, M. L. & Othman, S. F. Microscopic magnetic resonance elastography of traumatic brain injury model. J. Neurosci. Methods 201, 296–306 (2011).
4. Upadhyay, K. et al. Development and Validation of Subject-Specific 3D Human Head Models Based on a Nonlinear Visco-Hyperelastic Constitutive Framework. bioRxiv 1–27 (2021).
5. McGarry, M. D. J. et al. Multiresolution MR elastography using nonlinear inversion. Med. Phys. 39, 6388–6396 (2012).
6. Manduca, A. et al. MR Elastography: Principles, guidelines, and terminology. Magn. Reson. Med. 85, 2377–2390 (2021).
Figures
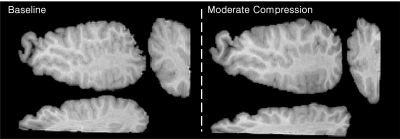
Figure 1. Representative T1-weighted magnitude images showing the bovine brain sample at baseline and at moderate compression. For both levels of pre-strain, three views of the brain tissue are provided: axial, sagittal, and coronal.
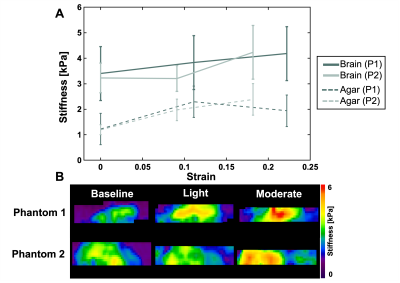
Figure 2. (A) Relationship between the apparent stiffness and the applied pre-strain for Phantom 1 (P1) and Phantom 2 (P2). The solid lines represent the outcomes in bovine brain tissue while the dashed lines represent the outcomes in the agar. (B) Representative stiffness maps for both phantoms at the three compression levels (baseline, light, and moderate). As applied pre-strain increased, both the brain tissue and the agar showed an increase in stiffness.
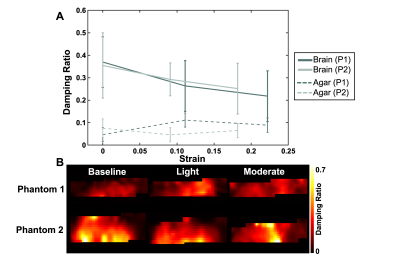
Figure 3. (A) Relationship between the damping ratio and the applied pre-strain for Phantom 1 (P1) and Phantom 2 (P2). The solid lines represent the outcomes in bovine brain tissue while the dashed lines represent the outcomes in agar. (B) Representative damping ratio maps for both phantoms at the three compression levels (baseline, light, and moderate). As the applied pre-strain was increased, the damping ratio of the brain tissue decreased. During compression the agar showed neither consistently higher nor lower damping ratio.
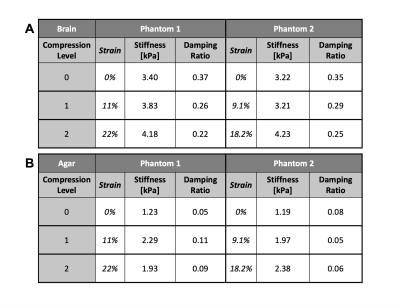
Figure 4. Table displaying stiffness and damping ratio values for (A) bovine brain tissue and (B) agar, for both phantoms at each level of applied pre-strain. Compression levels provided correspond to baseline (0), light (1), and moderate compression (2) and are quantified with the strain values provided. For brain tissue (A), we see an increase in apparent stiffness and a decrease in damping ratio across both phantoms. The agar (B) showed an increase in stiffness across both phantoms yet had inconsistent outcomes for the damping ratio.