5128
Cardiac Magnetic Resonance Feature Tracking for Detection of Acute Cardiac Allograft Rejection after Heart Transplantation1Department of Cardiology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China, HarBin, China, 2Department of Magnetic Resonance Imaging, Fuwai Hospital, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Beijing, China, 3Beihang University, Beijing, China
Synopsis
Keywords: Heart, Cardiovascular, Acute Cardiac Allograft Rejection
Acute cardiac allograft rejection is a major cause of morbidity and mortality after heart transplantation. Reliable non-invasive diagnostic techniques for acute cardiac allograft rejection (ACAR) are unavailable. Cardiac magnetic resonance feature tracking can detect global functional changes in the longitudinal, radial, and circumferential directions during the early stages of graft rejection. We found that cardiac magnetic resonance feature tracking (CMR-FT)-derived global longitudinal strain (GLS), global circumferential strain (GCS), and global radial strain (GRS) are safe, noninvasive, and gadolinium contrast-free parameters for ACAR diagnosis. CMR-FT may provide a method that can reduce the dependency on invasive EMBs after heart transplantation.Introduction
Heart transplantation remains the most effective treatment for patients with end-stage heart failure[1]. The first year after heart transplantation is associated with the highest risk of death; the leading causes of death include rejection, infection, graft failure, and multiple organ failure [2, 3]. Although the outcomes have significantly improved because of advances in immunosuppressive therapy, an alloimmune response can occur and cause acute cardiac allograft rejection (ACAR) which may lead to permanent damage to the transplanted heart. Therefore, early and accurate diagnosis of allograft rejection and prompt initiation of therapy are important. The current gold standard diagnostic modality for ACAR is an endomyocardial biopsy (EMB), about 10-15 EMBs are performed during the first year following a transplantation [4]. However, EMB is an invasive procedure that may result in serious complications, such as arrhythmia, thrombus formation, cardiac perforation, and pneumothorax [5-8]. Additionally, sampling error, inter-observer variability, and subsequent cost of histological analysis limit its clinical application[9-11]. Therefore, noninvasive alternatives are required to monitor acute allograft rejection in heart transplant recipients. Cardiac magnetic resonance (CMR) provides an insight into the heart function, morphology, and myocardial tissue characteristics. It is a significantly promising noninvasive screening tool for ACAR. CMR-FT technique can be used to assess cardiac deformation without additional CMR scan sequences by using standard cine CMR images . Recently, several studies have confirmed that CMR-FT GLS can predict major adverse cardiac events and all-cause mortality and has the prognostic value in heart transplantation recipients [12, 13]. Nevertheless, studies evaluating the CMR-FT strain technique for ACAR diagnosis are lacking. Therefore, this study aimed to determine the diagnostic role of CMR-FT strain parameters in heart transplant recipients.Methods
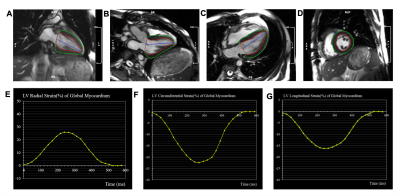
Sixty-one patients who underwent CMR within 24 hours before or after a routine endomyocardial biopsy were included in this study. CMR images were taken with 1.5-T & 3.0T scanners (Magnetom Avanto, Siemens, Germany; Ingenia CX, Philips Healthcare, the Netherlands) equipped with an 8-channel cardiac coil. Cine images included a stack of parallel short-axis and three long-axis views (2-, 3-, and 4-chamber) with a balanced steady-state free-precession. All strain parameters were obtained using a commercial software cvi42 (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada) by experienced radiologist with more than 5 years of training in cardiac imaging. Cine images were imported into the tissue tracking module of the cvi42. The endocardial and epicardial borders of the LV wall were demarcated semi-automatically on end-diastolic images (Figure 1). ACAR was categorized according to the International Society for Heart and Lung Transplantation (ISHLT) grade. CMR-FT parameters were compared using the endomyocardial biopsy findings. Univariable logistic regression was performed, and variables with P values < 0.10 in the univariable analysis included in subsequent multivariable logistic regression to predict the best diagnostic factors. Receiver operating characteristic (ROC) curve analysis was used to evaluate the ability of different strain parameters to detect rejection.Results
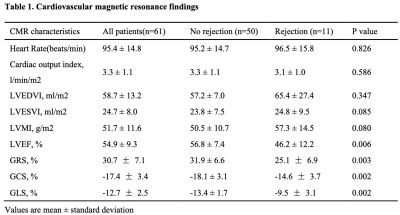
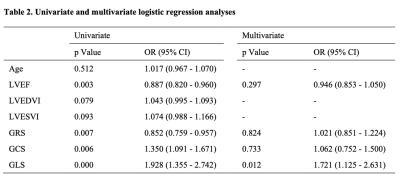
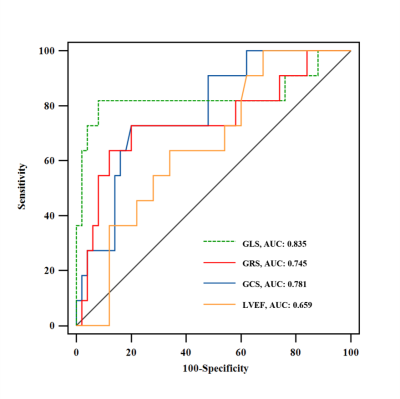
Of the 61 patients included, 50 (82%) with an ISHLT grade 0R or 1R were categorized into the no rejection group, while 11 (18%) with an ISHLT grade 2R were categorized into the rejection group. Strain analysis revealed significantly lower global longitudinal strain (GLS; -13.4±1.7% vs. -9.5±3.1%, P=0.002), global circumferential strain (GCS; -18.1±3.1% vs. -14.6±3.7%, P=0. 002) and global radial strain (GRS; 31.9±6.6% vs. 25.1±6.9%, P=0.003) in the rejection group than in the no rejection group (Table 1). GLS was found to be the independent predictor of acute rejection (p = 0.026; 95% confidence interval: 1.06 - 2.54) (Table 2). Receiver operating characteristic curve analysis revealed that the area under the curve (AUC) for GLS for detecting acute rejection was 0.835. The AUCs for GCS and GRS were 0.781 and 0.745, respectively (Figure 2).Discussion
CMR is becoming an important diagnostic tool for detecting acute rejection in heart transplant recipients [14]. In this retrospective study, we utilized CMR-FT-derived strain parameters to supplement the noninvasive diagnosis of acute rejection in cardiac transplant recipients. The strain parameters including GCS, GLS, and GRS were significantly higher in recipients in the no rejection group than in those in the rejection group. ROC curve analysis revealed that the CMR-FT-derived GLS, GCS, and GRS had good predictability for rejection. Studies have demonstrated that speckle-tracking echocardiography-derived strain parameters might be useful diagnostic tools for detecting acute cellular rejection in cardiac transplant recipients [15-17]. A meta‐analysis revealed that the mean GLS and GCS in healthy participants were -20.1% and -23%, respectively [18]. Our findings showed that even with no rejection, the GLS (-14.5 ± 1.3) and GCS (-18.6 ± 2.6) values were significantly decreased in the recipients when compared to healthy individuals. These findings are consistent with those of other comparable studies [19-21]. One reason could be illustrated that the transplanted hearts were damaged due to various reasons, such as ischemia, hypoxia, denervation, calcium overload, immune attack, and reperfusion injury. Thus, the “normal” strain values in transplant recipients without rejection are lower than those in healthy participants and remain constant unless an allograft rejection occurs [22]. The deterioration of myocardial strain could be an early indication of the clinical need for further investigation and adjustments in the treatment.Conclusions
CMR-FT-derived GLS, GCS, and GRS are safe, noninvasive, and gadolinium contrast-free parameters for detecting ACAR.Acknowledgements
NoneReferences
1. Shah K, Kittleson M, Kobashigawa J. Updates on Heart Transplantation. Current heart failure reports. 2019;16(5):150-6.2. Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30(10):1078-94.
3. Stewart S, Winters G, Fishbein M, Tazelaar H, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(11):1710-20.
4. Hamour IM, Burke MM, Bell AD, Panicker MG, Banerjee R, Banner NR. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation. 2008;85(7):969-74.
5. From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86(11):1095-102.
6. Pophal SG, Sigfusson G, Booth KL, Bacanu S-A, Webber SA, Ettedgui JA, et al. Complications of endomyocardial biopsy in children. Journal of the American College of Cardiology. 1999;34(7):2105-10.
7. Rali AS, Sami F, Sauer A, Shah Z. Right Coronary Artery to Coronary Sinus Fistula Post Endomyocardial Biopsy: A Case Report of a Rare Complication. Case Rep Cardiol. 2020;2020:7914737.
8. Veress G, Bruce CJ, Kutzke K, Click RL, Scott CG, Oh JK, et al. Acute thrombus formation as a complication of right ventricular biopsy. J Am Soc Echocardiogr. 2010;23(10):1039-44.
9. Angelini A, Andersen CB, Bartoloni G, Black F, Bishop P, Doran H, et al. A web-based pilot study of inter-pathologist reproducibility using the ISHLT 2004 working formulation for biopsy diagnosis of cardiac allograft rejection: the European experience. J Heart Lung Transplant. 2011;30(11):1214-20.
10. Crespo-Leiro MG, Zuckermann A, Bara C, Mohacsi P, Schulz U, Boyle A, et al. Concordance among pathologists in the second Cardiac Allograft Rejection Gene Expression Observational Study (CARGO II). Transplantation. 2012;94(11):1172-7.
11. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710-20.
12. Shenoy C, Romano S, Hughes A, Okasha O, Nijjar PS, Velangi P, et al. Cardiac Magnetic Resonance Feature Tracking Global Longitudinal Strain and Prognosis After Heart Transplantation. JACC Cardiovasc Imaging. 2020.
13. Clemmensen TS, Eiskjaer H, Logstrup BB, Ilkjaer LB, Poulsen SH. Left ventricular global longitudinal strain predicts major adverse cardiac events and all-cause mortality in heart transplant patients. J Heart Lung Transplant. 2017;36(5):567-76.
14. Dolan RS, Rahsepar AA, Blaisdell J, Suwa K, Ghafourian K, Wilcox JE, et al. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection After Heart Transplantation. JACC Cardiovasc Imaging. 2019;12(8 Pt 2):1632-41.
15. Elkaryoni A, Altibi AM, Khan MS, Okasha O, Ellakany K, Hassan A, et al. Global longitudinal strain assessment of the left ventricle by speckle tracking echocardiography detects acute cellular rejection in orthotopic heart transplant recipients: A systematic review and meta-analysis. Echocardiography. 2020;37(2):302-9.
16. Ciarka A, Cordeiro F, Droogne W, Van Cleemput J, Voigt JU. Speckle-tracking-based global longitudinal and circumferential strain detect early signs of antibody-mediated rejection in heart transplant patients. Eur Heart J Cardiovasc Imaging. 2021.
17. Du GQ, Hsiung MC, Wu Y, Qu SH, Wei J, Yin WH, et al. Three-Dimensional Speckle-Tracking Echocardiographic Monitoring of Acute Rejection in Heart Transplant Recipients. J Ultrasound Med. 2016;35(6):1167-76.
18. Vo HQ, Marwick TH, Negishi K. MRI-Derived Myocardial Strain Measures in Normal Subjects. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):196-205.
19. Chamberlain R, Scalia GM, Shiino K, Platts DG, Sabapathy S, Chan J. Diastolic strain imaging: a new non-invasive tool to detect subclinical myocardial dysfunction in early cardiac allograft rejection. Int J Cardiovasc Imaging. 2020;36(2):317-23.
20. Saleh HK, Villarraga HR, Kane GC, Pereira NL, Raichlin E, Yu Y, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J Heart Lung Transplant. 2011;30(6):652-8.
21. Ingvarsson A, Werther Evaldsson A, Waktare J, Nilsson J, Smith GJ, Stagmo M, et al. Normal Reference Ranges for Transthoracic Echocardiography Following Heart Transplantation. J Am Soc Echocardiogr. 2018;31(3):349-60.
22. Pichler P, Binder T, Höfer P, Bergler-Klein J, Goliasch G, Lajic N, et al. Two-dimensional speckle tracking echocardiography in heart transplant patients: three-year follow-up of deformation parameters and ejection fraction derived from transthoracic echocardiography. European heart journal Cardiovascular Imaging. 2012;13(2):181-6.Figures