5125
CNNT denoising for cine imaging at 0.55T with higher acceleration rates1National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: Heart, Machine Learning/Artificial Intelligence
Low field MRI systems are promising to increase the accessibility of cardiac MRI, at the expense of lower SNR. Here, we present the application of a g-factor-savvy denoising for cine imaging at 0.55T to increase useable acceleration rate. We used a model that combines convolutional neural networks and transformer model. The denoising network uses complex 2D+time images in SNR-units and g-factor maps as inputs and was trained with 3T data. The percent mean myocardial SNR gain at 0.55T across 9 healthy volunteers was 97±31% (R=2) and 122±22% (R=3), with no indication of overt temporal or spatial smoothing.Introduction
Low magnetic fields of 0.35T and 0.55T have demonstrated promise for clinical CMR [1, 2]. Low field systems may potentially increase the accessibility of cardiac MRI by reducing the hardware and operation cost. Low field MRI offers inherently lower SNR, and commercial low field systems operate with reduced gradient performance and fewer receiver channels. These hardware limitations restrict rapid imaging capabilities by enforcing longer TRs and lower accelerations rates (typically R=1 or 2).Deep learning offers the capability to denoise images, but many denoising approaches are naïve to the non-uniformity of noise amplification based on coil geometry, g-factor, and underlying SNR. Moreover, most algorithms require retraining with large datasets for each field strength. Here, we present the application of a g-factor-savvy denoising for cine imaging at 0.55T to increase useable acceleration rate.
Methods
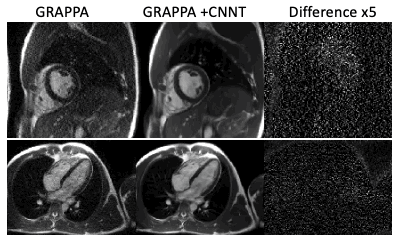
We used a model that utilizes a new network architecture combining convolutional neural networks (CNNs) and the more recent transformer model. The linear transformation in the standard attention module was replaced with convolution layers, to significantly reduce the computation for the input 2D+time image series. The attention mechanism is still maintained so every output is a weighted average of all inputs, with the attention weights computed adaptively to the specific input. The novel CNN transformer denoising model (CNNT) [3] used both complex 2D+time images in SNR-units [4] and g-factor maps as inputs. The model was trained on 3T retro-gated cine data, and applied to 0.55T gated cine without retraining. The denoising model was deployed inline on the scanner using Gadgetron InlineAI [5].Images were acquired on a commercial 0.55T MRI system (MAGNETOM Free.Max, Siemens Healthcare, Erlangen, Germany). Breath-held retrospectively ECG-gated bSSFP cine images were acquired using acceleration rates R=2, 3, and 4 (parameters in Table 1). Notably, the acquired temporal resolution was 42.6ms for R = 2 (23 acquired cardiac phases for 60bpm heart rate), compared with 37.9ms (26 phases) for R = 3 and 31.9ms (31 phases) for R = 4. Regardless of acquired cardiac phases, the data was interpolated to 30 phases.
Imaging was performed on 9 healthy volunteers and two patients with known wall-motion abnormality from chronic myocardial infarction. ECG-gating used an external physiological monitoring system (Tesla, Surgical Tools Inc, Bedford VA). SNR gain was measured in the septum between the original and denoised images in a midventricular short axis slice, by the pseudo-replica method [4].
Results
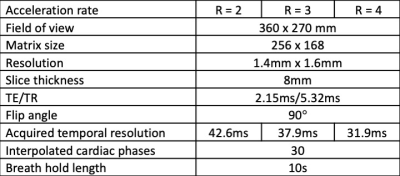
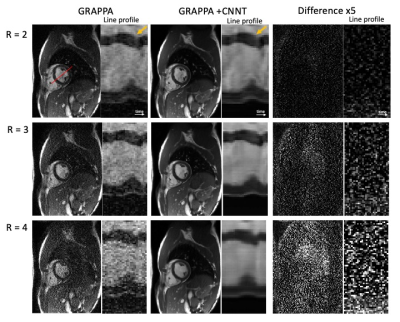
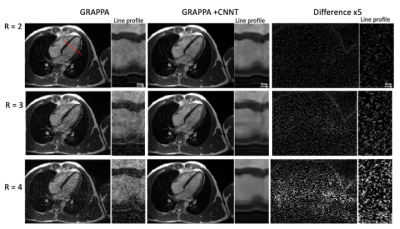
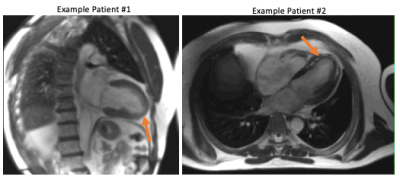
Figures 1 and 2 provide example images, and line profiles through time, from short axis and long axis slices using acceleration rates R=2, 3, and 4. The denoising of the CNNT network can be appreciated. Acceleration rate R=2 acquired only 23 phases per heartbeat and myocardial blurring was evident following the interpolation to 30 frames. The CNNT denoising enabled reliable use of acceleration rate R=3 for cine imaging in all subjects, whereas R=4 may start to reach the limit of the model where signal can be distinguished from noise. Figure 3 provides animated cine images with acceleration rate R = 3 for short axis and 4 chamber-view. The wall-motion abnormalities in two patients were visible following CNNT denoising (with R = 3), as illustrated in animated Figure 4. The percent mean myocardial SNR gain across 9 healthy volunteers was 97±31% (R=2), 122±22% (R=3), and 476±130% (R=4).Discussion and Conclusion
AI-based denoising demonstrated improved image SNR for cine at 0.55T. Importantly, this CNNT method uses the SNR-scaled image reconstruction and g-factor maps for improved generalization, and the CNNT model did not require retraining for application at 0.55T. The myocardial anatomy and wall motion were better delineated in the denoised images, with difference images and temporal profiles showing no visible structures. Improved acceleration rate can be traded for improved acquired temporal resolution or shorter breath-holds, and may reduce the hardware requirements for rapid cardiac imaging at low field.Acknowledgements
This work was supported by the NHLBI DIR (Z01-HL006213, Z01-HL006257). We thank Scott Baute, Amelia Nargozian and Haiyan Wang for their assistance with this project. We would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement between NHLBI and Siemens Healthcare.References
1. Simonetti, O.P. and R. Ahmad, Low-Field Cardiac Magnetic Resonance Imaging: A Compelling Case for Cardiac Magnetic Resonance's Future. Circ Cardiovasc Imaging, 2017. 10(6).
2. Bandettini, W.P., et al., A comparison of cine CMR imaging at 0.55 T and 1.5 T. J Cardiovasc Magn Reson, 2020. 22(1): p. 37.
3. Rehman, A., et al., Convolutional neural network transformer (CNNT) for free-breathing real-time cine imaging. , in Artificial Intelligence in Cardiovascular Magnetic Resonance Imaging - A Joint Summit of the EACVI and SCMR. 2022.
4. Kellman, P. and E.R. McVeigh, Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med, 2005. 54(6): p. 1439-47.5. Xue, H., et al., Gadgetron Inline AI : Effective Model inference on MR scanner, in Proceedings of the International Society for Magnetic Resonance in Medicine annual meeting. 2019. p. 4837.
Figures