5117
Progress toward a ZTE-based silent and motion-robust protocol for pediatric neuroimaging1Radiology, University of Iowa, Iowa City, IA, United States, 2Electrical and Computer Engineering, University of Iowa, Iowa City, IA, United States, 3Champaign Imaging, LLC, Shoreview, MN, United States
Synopsis
Keywords: Neuro, Pulse Sequence Design, silent imaging
In this work, we present progress in developing a ZTE-based silent and motion-robust neuroimaging protocol using intermittent magnetization preparation for generation of standard imaging contrasts including T1w and T2w.Purpose
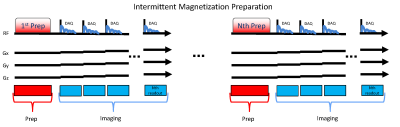
Imaging pulse sequences typically used in neuroimaging studies rely on conventional Cartesian encoding methods. However, these approaches are known to generate considerable acoustic noise due to high imaging gradient slew rates and amplitudes. The loud acoustic noise can present significant challenges in studying sensitive populations such as developmental disorders in pediatrics. Approaches have been proposed to address the loud acoustic noise typically associated with MRI including hardware and acquisition approaches such as shaped gradient pulses [1] and zero-echo time (ZTE) [2] methods. Radial sampling methods have been demonstrated to provide motion robustness [3] or even correction [4], making the radial acquisition ZTE approach appealing. To utilize the ZTE sampling trajectory across a variety of image weightings, intermittent magnetization preparation approaches are needed to generate the range of imaging contrasts needed (e.g. T1w and T2w). In this abstract, we present our progress in developing magnetization prepared ZTE imaging protocols.Methods
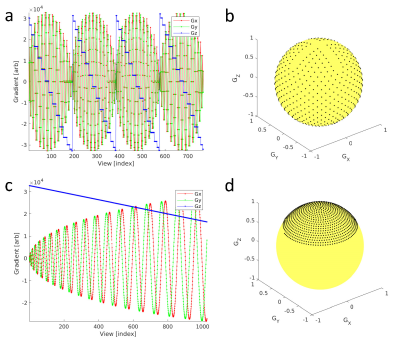
3D radial ZTE acquisition parameters included 25.6cm isotropic FOV, 256 acquired matrix isotropic, +/-31.25kHz BW. An intermittent magnetization preparation pulse (T1w: adiabatic inversion pulse or T2w: 4x180o composite RF pulses in a MLEV scheme) was applied once per collection of each segmented readout block consisting of 192 ZTE FID radial lines (Fig. 1). The order of angular k-space lines was optimized to allow coverage of angles from -z to +z within each 192 FID segmented readout [5] compared to more conventional approaches that acquire place the angles in a single spiral [6] (Fig. 2) to facilitate use of angular undersampling and interleaved projection angles. A total of 32,640 and 48,960 angles (6.3x and 4.2x undersampled) were sampled for scan times of 6:46 (min:sec) and 6:35 (min:sec) for T1w and T2w respectively. The readout was 2x oversampled, with proportional bandwidth increase, covering a nominal FOV of twice the proscribed FOV, while adding no additional scan time [7]. For immediate preview, reconstruction of the 3D radial data was accomplished with 1.25 oversampled optimal Kaiser-Bessel kernel [8] and Pipe-Menon sample density compensation [9]. Conventional Cartesian imaging was performed using 3D gradient echo T1w MPRAGE and a 3D T2w FSE CUBE. Cartesian T1w scan parameters included FOV 25.6cm, 256x256x180 acquired matrix, +/-31.25kHz BW and 5:13 (min:sec) scan time. Cartesian T2w scan parameters included FOV 25.6cm, 256x256x180 acquired matrix, +/-62.5kHz BW and 5:10 (min:sec) scan time. A normal healthy volunteer provided written consent and was imaged in this IRB approved study. Imaging was performed on a 3T clinical MRI (Premier, GE Healthcare, Waukesha, WI) using a 48ch receive array head coil (GE Healthcare). Acoustic noise measurements were performed within the MRI bore at the location of the head coil during individual pulse sequences.Results
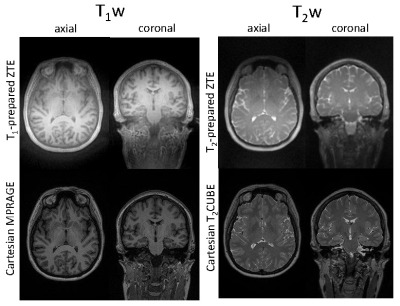
Axial and coronal reformats of the 3D image volumes show similar T1w and T2w image contrast using undersampled ZTE compared to the conventional Cartesian gradient echo and FSE standards (Fig 3). Further, acoustic noise measurements during scanning indicated that the increased gradient ramping of the interleaved radial angle acquisition ZTE based approach only increase the maximum sound pressure level (SPL) by 5dBc compared the conventional single-spiral 'silent' ZTE view order (upper limits of 82dBc and 77dBc maximum SPL, respectively), thus keeping it considerably lower than conventional the Cartesian scans (lower limit of 106dBc maximum SPL).Conclusions
In this abstract, we present progress to date on developing a ZTE-based silent and motion-robust neuroimaging protocol. A segmented k-space ZTE readout radial readout scheme was utilized to allow intermittent magnetization preparation for generation of standard imaging contrasts including T1w and T2w. The interleaved HEALPix radial view-order scheme [5] provides coverage from +z to -z poles within each 192 ZTE FIDs making the trajectory well suited for undersampling and parallel imaging compressed sensing as well as interleaved acquisitions and motion correction approaches [10-13]. Although the larger angular displacements between adjacent FIDs in time results in increased gradient ramping and some increase in acoustic noise, the SPL levels remained well below conventional Cartesian based imaging protocols. Future work will aim to increase the variety of imaging contrasts to better accommodate more comprehensive imaging protocols.Acknowledgements
This work was funded in part by NIH R43MH122028. JHH and VAM received salary support from NIH P50HD103556 and the data for this project was collected on an instrument funded by NIH S10OD025025. We thank Stephen Otto for project management and logistics related to this work.References
[1] Segbers, M.; Sierra, C. V. R.; Duifhuis, H. & Hoogduin, J. M. (2010). Shaping and timing gradient pulses to reduce MRI acoustic noise., Magn Reson Med 64 : 546-553. PMID: 20665798
[2] Alibek, S.; Vogel, M.; Sun, W.; Winkler, D.; Baker, C. A.; Burke, M. & Gloger, H. (2014). Acoustic noise reduction in MRI using Silent Scan: an initial experience., Diagn Interv Radiol 20 : 360-363. PMID: 24808439
[3] Glover, G. H. & Pauly, J. M. (1992). Projection reconstruction techniques for reduction of motion effects in MRI., Magn Reson Med 28 : 275-289. PMID: 1461126
[4] Welch, E. B.; Rossman, P. J.; Felmlee, J. P. & Manduca, A. (2004). Self-navigated motion correction using moments of spatial projections in radial MRI., Magn Reson Med 52 : 337-345. PMID: 15282816
[5] Corum, C. A.; Kruger, S. & Magnotta, V. A. (2020). HEALPix View-order for 3D+time Radial Self-Navigated Motion-Corrected ZTE MRI, 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) : 0409. PMID: http://arxiv.org/pdf/1910.10276v2
[6] Wong, S.T.S.; Roos, M.S. (1994) A strategy for sampling on a sphere applied to 3D selective RF pulse design, Magn. Reson. Med. 32: 778–784, https://doi.org/10.1002/mrm.1910320614.
[7] Zhang, S.; Block, K. T. & Frahm, J. (2010). Magnetic resonance imaging in real time: advances using radial FLASH., Journal of magnetic resonance imaging : JMRI 31 : 101-109. PMID: 19938046
[8] Beatty, P. J.; Nishimura, D. G. & Pauly, J. M. (2005). Rapid gridding reconstruction with a minimal oversampling ratio, Medical Imaging, IEEE Transactions on 24 : 799 - 808. PMID: 15959939
[9] Pipe, J. G. & Menon, P. (1999). Sampling density compensation in MRI: rationale and an iterative numerical solution., Magn Reson Med 41 : 179-186. PMID: 10025627
[10] Pipe, J. G. (1999). Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging, Magnetic Resonance in Medicine 42 : 963-969. PMID: 10542356
[11] Hedley, M.; Yan, H. & Rosenfeld, D. (1991). Motion artifact correction in MRI using generalized projections., IEEE Trans Med Imaging 10 : 40-46. PMID: 18222798
[12] Shankaranarayanan, A.; Wendt, M.; Lewin, J. S. & Duerk, J. L. (2001). Two-step navigatorless correction algorithm for radial k-space MRI acquisitions, Magnetic Resonance in Medicine 45 : 277-288. PMID: 11180436
[13] Kecskemeti, S.; Samsonov, A.; Velikina, J.; Field, A. S.; Turski, P.; Rowley, H.; Lainhart, J. E. & Alexander, A. L. (2018). Robust Motion Correction Strategy for Structural MRI in Unsedated Children Demonstrated with Three-dimensional Radial MPnRAGE., Radiology 289 : 509-516. PMID: 30063192
Figures