5116
Feasibility of detecting subvoxel deformation in intervertebral disc using quantitative ultrashort echo time (UTE) techniques1Department of Radiology, University of California, San Diego, La Jolla, CA, USA, San Diego, CA, United States, 2Radiology Service, Veterans Affairs San Diego Healthcare System, San Diego, La Jolla, CA, USA, San Diego, CA, United States, 3Department of Orthopedic Surgery, University of California, San Diego, La Jolla, CA, USA, San Diego, CA, United States
Synopsis
Keywords: Skeletal, Magnetization transfer, Intervertebral disc
Quantitative ultrashort echo time (UTE) MRI can be used for quantitative assessment of intervertebral discs (IVDs). It is hypothesized that the investigation of quantitative UTE MRI properties of IVD under mechanical loading may highlight the affected regions of IVD by diseases and injuries. We investigated the feasibility of using UTE-T1, UTE-Adiab-T1ρ, and UTE-MT measures for detecting the IVD deformation under loading. T1 and T1 ρ decreased in IVDs under loading while MMF from UTE-MT modeling as an index for collagen content increased by loading. This study highlights the potential of UTE-MRI to detect subvoxel deformations in IVDs caused by loading.Introduction
Although the precise etiology of low back pain is not fully understood, intervertebral disc (IVD) degeneration is recognized as one of the major contributors to the condition. Magnetic resonance imaging (MRI) has been the most promising modality in medical imaging to evaluate IVDs (1). Conventional MRI sequences, such as the T2-weighted fast spin echo (T2w-FSE), are highly effective for evaluating IVD morphological changes. Quantitative MRI techniques, including measurement of T2, T1ρ, magnetization transfer, and diffusion metrics, offer promising avenues for the compositional and microstructural evaluation of IVD (2). Spine MRI imaging traditionally takes place with the patient in non-weight-bearing positions, which is not optimal for the detection of IVD degeneration during early-stage diseases and minor injuries. It is hypothesized that the mechanical properties of IVD may vary at an early stage of the disease before any manifestation of gross morphological changes. Investigating quantitative MRI properties of IVD under mechanical loading may highlight the affected regions of IVD by diseases and injuries. T1, Adiabatic T1r and magnetization transfer (MT) modeling combined with ultrashort echo time (UTE) are three recently developed quantitative techniques suggested for IVD imaging as it contains a considerable amount of highly organized collagen fibers possessing short T2 values (3–5). Moreover, these techniques have shown low sensitivity to the magic angle effect (insensitive to tissue orientation in the scanner) (6–9)which suggests them as promising quantitative MRI techniques for investigating IVD under mechanical loading (10–12). UTE-MT modeling provides multiple parameters, including macromolecular fraction (MMF), macromolecular relaxation time (T2mm), and exchange rates. This study aimed to investigate the feasibility of using UTE-T1, UTE-Adiab-T1ρ, and UTE-MT measures for detecting IVD deformation under loading.Methods
A fresh frozen lumber spine specimen for a 34-year-old-male donor was provided by UCSD anatomical service lab. L3 and L5 level vertebrae were cut using a diamond blade band saw to include two IVDs in this feasibility study (L3-L4 and L4-L5 IVDs). The specimen was wrapped and sealed using absorbent pads and placed into an in-house designed loading device employing six plastic springs (LL100125U40G, Lee Spring, NY). The compression load was adjustable manually using four nylon screws. The specimen was scanned on a clinical 3T MR scanner (MR750, GE Healthcare Technologies, WI) in the sagittal plane using a brain coil before loading and under 20 kgf load while placed parallel to B0. The following three imaging protocols were performed: A) 3D-UTE-cones with variable flip angles (FA=5, 10, 20, and 30, TR=18 ms) for T1 measurement as a prerequisite for MT modeling and UTE-Adiab-T1ρ fitting. T1 values were modified after correcting for B1 inhomogeneity(3). (B) 3D-UTE-MT-cones with two saturation pulse powers (q=350°and 750°) and five frequency offsets (Df=2, 5, 10, 20, and 50 kHz) (5) for MT modeling, and C) 3D UTE-Adiab-T1r sequences with three different spin-locking times (TSLs) (TSL= 0, 24, and 48 ms) for T1ρ measurement (4). Other imaging parameters included: FOV=14cm, matrix=240×240, slice thickness=3 mm, 16 slices. The total scan time was 40 mins. T1, T1ρ, MMF maps, and their average values were calculated for the two IVDs and compared between pre-loading and underloading datasets.Results
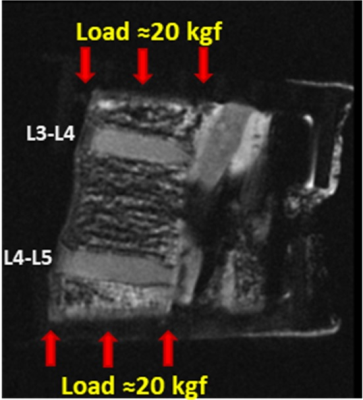
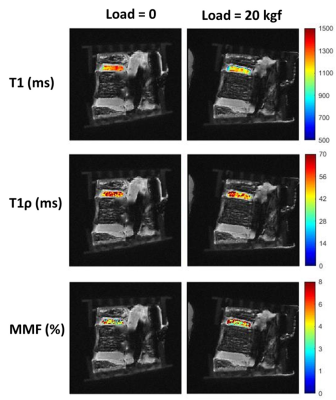
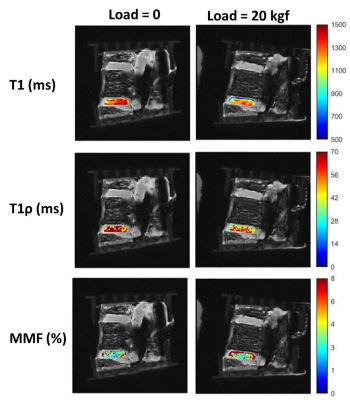
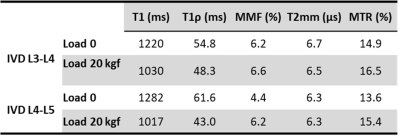
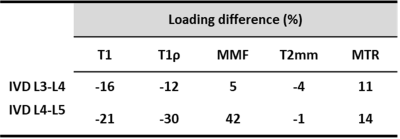
Figure 1 shows a schematic depiction of the axial load applied by the loading device (employed 6 plastic springs not seen in this image) on the sectioned lumbar spine. The schematic load arrows are shown on a UTE-MRI image acquired in the sagittal plane illustrating L3-L4 and L4-L5 IVDs. Figures 2 and 3 illustrate UTE-T1, UTE-Adiab-T1ρ, and MMF maps overlayed on the sagittal UTE-MR images before loading (left column) and under 20 kgf load (right column) for the L3-L4 and L4-L5 IVDs, respectively. Obviously, T1 and T1ρ decreased by loading while MMF increased by loading. The average UTE-T1, UTE-Adiab-T1ρ, and MMF values in IVDs before loading and under loading are presented in Figure 4 (Table 1). The average percentage differences caused by loading in UTE measures with IVDs are presented in Figure 5 (Table 2). T1, T1ρ, and T2mm decreased by loading while MMF and MTR increased by loading in both IVDs. L4-L5 experienced higher percentage differences caused by loading. The highest differences were found in MMF.Discussion
The feasibility of using UTE-T1, UTE-Adiab-T1ρ, and UTE-MT measures for detecting the IVD deformation under mechanical loading was investigated in two human IVDs. The decreasing trend of T1, T1ρ, and T2mm by loading and the increasing trend of MMF and MTR may imply an outward flow of water from IVDs caused by loading. Moreover, the deformation caused by loading results in a denser collagenous matrix in IVD as indicated by higher MMF. IVD deformation was likely higher for L4-L5 level due to the potential non-uniformity and misalignment in axial load application.Acknowledgements
The authors acknowledge grant support from the National Institutes of Health (R01AR068987, R01AR062581, R01AR075825, K01AR080257, R01AR079484, and 5P30AR073761), Veterans Affairs Clinical Science R&D (I01CX001388 and I01CX000625), and GE Healthcare.References
1. Cousins JP, Haughton VM. Magnetic resonance imaging of the spine. Journal of the American Academy of Orthopaedic Surgeons 2009;17:22–30 doi: 10.1007/978-3-540-74504-4_3.
2. Mwale F, Iatridis JC, Antoniou J. Quantitative MRI as a diagnostic tool of intervertebral disc matrix composition and integrity. European Spine Journal 2008;17:432–440 doi: 10.1007/s00586-008-0744-4.
3. Ma YJ, Zhao W, Wan L, et al. Whole knee joint T1 values measured in vivo at 3T by combined 3D ultrashort echo time cones actual flip angle and variable flip angle methods. Magn Reson Med 2019;81:1634–1644 doi: 10.1002/mrm.27510.
4. Ma Y, Carl M, Searleman A, Lu X, Chang EY, Du J. 3D adiabatic T1ρprepared ultrashort echo time cones sequence for whole knee imaging. Magn Reson Med 2018;80:1429–1439 doi: 10.1002/mrm.27131.
5. Ma Y, Chang EY, Carl M, Du J. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn Reson Med 2017;79:692–700 doi: 10.1002/mrm.26716.
6. Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissues. Magn Reson Med 1994;32:592–601 doi: 10.1002/mrm.1910320508.
7. Ma Y, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed 2016;29:1546–1552 doi: 10.1002/nbm.3609.
8. Wu M, Kasibhatala A, Ma Y jun, Jerban S, Eric Y. Chang, Du J. Magic angle effect on adiabatic T1ρ imaging of the Achilles tendon using 3D ultrashort echo time cones trajectory. NMR Biomed 2020;30:1–10.
9. Wu M, Ma Y, Kasibhatla A, et al. Convincing evidence for magic angle less‐sensitive quantitative T 1ρ imaging of articular cartilage using the 3D ultrashort echo time cones adiabatic T 1ρ (3D UTE cones‐AdiabT 1ρ ) sequence. Magn Reson Med 2020;1:1–8 doi: 10.1002/mrm.28317.
10. Jerban S, Chang EricYEY, Du J. Magnetic resonance imaging (MRI) studies of Knee joint under mechanical loading: review. Magn Reson Imaging 2020;65:27–36 doi: https://doi.org/10.1016/j.mri.2019.09.007.
11. Jerban S, Kasibhatla A, Ma Y, et al. Detecting Articular Cartilage and Meniscus Deformation Effects Using Magnetization Transfer Ultrashort Echo Time ( MT-UTE ) Modeling during Mechanical Load Application : Ex Vivo Feasibility Study. Cartilage 2020;8:1–10 doi: 10.1177/1947603520976771.
12. Jerban S, Ma Y, Kasibhatla A, et al. Ultrashort echo time adiabatic T1ρ (UTE-Adiab-T1ρ) is sensitive to human cadaveric knee joint deformation induced by mechanical loading and unloading. Magn Reson Imaging 2021;80:98–105 doi: 10.1016/j.mri.2021.04.014.
Figures