5114
Robust assessment of macromolecular fraction in muscle with differing fat fraction using ultrashort echo time magnetization transfer modeling1Radiology, University of California, San Diego, San Diego, CA, United States, 2Radiology Service, Veterans Affairs San Diego Healthcare System, San Diego, La Jolla, CA, USA, San Diego, CA, United States
Synopsis
Keywords: Muscle, Magnetization transfer, Collagen Content; Fat Content
Magnetization transfer (MT) MR imaging can indirectly characterize the spatial distribution of the relative contents of the macromolecular and water proton pools (MMF) of skeletal muscles. Fat presence in muscle has always been a source of concern in MMF calculation where some studies have reported significant underestimation of MMF for muscles with a considerable fat fraction (FF). We investigated the impact of FF on MMF in muscle/fat phantoms using an ultrashort echo time (UTE) MT model after T1 compensation. MMF demonstrated a relatively robust value with under 5% and 20% changes for FF increases up to 30 and 45%, respectively.INTRODUCTION
Developing robust quantitative MRI-based evaluation of skeletal muscles has been of great interest to several research groups (1–3). Magnetization transfer (MT) imaging combined with ultrashort echo time (UTE) MRI has been recently introduced as a technique to indirectly characterize the spatial distribution of the relative contents of the macromolecular and water proton pools of biological tissues (4,5). Targeting skeletal muscle evaluations, UTE-MRI in MT modeling may help improve the evaluation of the myotendinous junction and muscles with fibrotic tissues which possess short T2 values. Using MT techniques, a high-power saturation RF pulse (such as Fermi type pulse) is used with a defined frequency offset from the water protons’ resonance frequency to saturate mainly protons in collagenous muscle fibers. The saturated magnetization transfers from the collagenous fibers to water protons that can be detected by UTE-MRI. The magnitude of the transferred saturation to water protons correlates with the quantity of collagen protons relative to water protons in the tissue. UTE-MRI MT modeling is an insensitive technique to the magic angle effect (4,6) and provides multiple parameters, including macromolecular fraction (MMF), macromolecular relaxation time (T2mm), and exchange rates tissues (4,5). Accurate MT modeling requires B1 correction and T1 compensation (7). Although fat in theory does not participate in MT phenomena, fat presence in muscle has always been a source of concern in MMF calculation and several studies have reported significant underestimation of MMF for muscles with a considerable fat fraction (FF). This study aimed to investigate the impact of FF in estimated MMF in bovine muscle phantoms embedded in pure fat (lard).METHODS
Fresh cuts of lean bovine muscles and pure pork fat (lard) were obtained. The muscle cuts were visually examined to avoid the inclusion of obvious interfascicular fat in the prepared muscle specimen. Two muscle sections were placed in a cylindrical plastic container. Next, melted lard (was melted at 60°C and then cooled down to near room temperature) was poured into the container containing two muscle sections. The muscle-fat phantom was kept at room temperature until the lard reached its stable solid state. The container was topped with tap water to ensure performing MR imaging at the water peak frequency. Muscle/Fat phantom was imaged using UTE-MRI sequences on a 3T clinical scanner (MR750, GE). To measure T1 as a prerequisite for the two-pool UTE-MT modeling, actual flip angle - variable flip angle (AFI-VFA) sequence (AFI: TE=0.032ms, TRs=20,100ms, FA=45˚; VFA: TE=0.032ms, TR=20ms, FAs=5, 12, 24˚) was performed (8). A 3D-UTE-Cones-MT sequence (pulse power=500°, 1000°, and 1500°; frequency offset=2, 5, 10, 20, and 50kHz; FA=7˚; 11 spokes per MT preparation) was performed for the two-pool MT modeling (5,9). Field of view, matrix dimension, slice thickness, and total scan time were 12 cm, 192×192, 4mm, and 25 mins, respectively. UTE-MRI analyses were performed initially within seven regions of interest (ROIs) covering only muscle to calculate T1, MT ratio, MMF, and T2mm. Each ROI was then gradually expanded by only adding the neighboring pure fat voxels (lard) while the originally included muscle voxels were kept intact.RESULTS
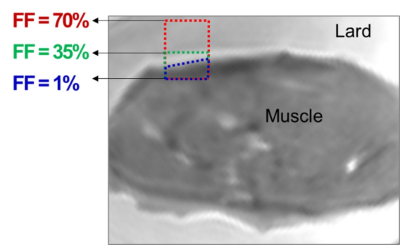
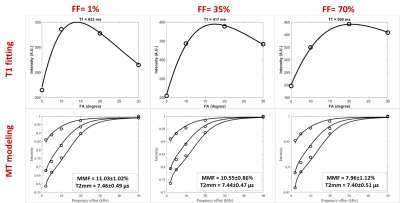
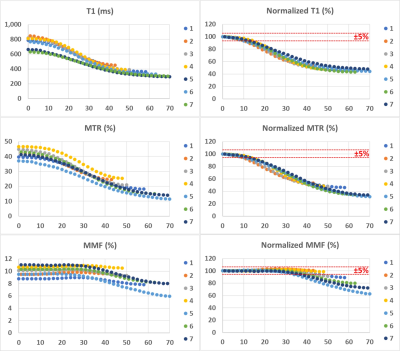
Figure 1 demonstrates a T1 weighted UTE MRI image of the bovine muscle specimen embedded in lard (i.e., 100% fat content, FF). An exemplary ROI is highlighted in blue box with initial FF of 1 % which expanded to an ROI with 70% FF by adding the neighboring lard voxels (red box) while the muscle compartment of the expanding ROIs is constant. Figure 2 depicts the T1 fittings (single component exponential model) on a variable FA dataset and two-pool MT models using a super-Lorentzian function over variable off resonance frequencies for three different power levels (500°, 1000°, and 1500°) within the three exemplary ROIs shown in Figure 1 (FF = 1, 35, and 70%). T1, MMF, and T2mm demonstrated a decreasing trend by FF increase in muscle/lard ROIs. The variation of the absolute and normalized T1, MTR, and MMF measures versus FF are shown in in Figure 3 for the seven selected muscle ROIs. T1 and MTR decreased by more than 5% for FF increased above 10%. For FF above 45%, T1 and MTR decreased to the around 50% of their original values. MMF demonstrated a relatively robust value with under 5% and 20% changes for FF increases up to 30 and 45%, respectively.DISCUSSION
The impact of FF in MMF estimations was investigated using a muscle/lard phantom. Estimated MMF from UTE-MT modeling was very robust and showed under 5% changes for muscle/fat ROIs with FF up to 30%. This study highlighted the potential of the two-pool UTE-MT modeling with T1 compensation method for robust collagen content estimation as a measure of muscle quality, while remaining insensitive to fat infiltration up to moderate levels.Acknowledgements
The authors acknowledge grant support from the National Institutes of Health (R01AR068987, R01AR062581, R01AR075825, K01AR080257, R01AR079484, and 5P30AR073761), Veterans Affairs Clinical Science R&D (I01CX001388 and I01CX000625), and GE Healthcare.References
1. Ke Lia, Richard D. Dortcha, E. Brian Welcha, et al. Multi‐parametric MRI characterization of healthy human thigh muscles at 3.0 T–relaxation MTat water and DTI - NMRB 2014. NMR Biomed 2014;27:1070–1084.
2. Li K, Dortch RD, Kroop SF, et al. A rapid approach for quantitative magnetization transfer imaging in thigh muscles using the pulsed saturation method. Magn Reson Imaging 2015;33:709–717 doi: 10.1016/j.mri.2015.03.003.
3. Romero IO, Sinha U. Magnetization transfer saturation imaging of human calf muscle: Reproducibility and sensitivity to regional and sex differences. Journal of Magnetic Resonance Imaging 2019;50:1227–1237 doi: 10.1002/jmri.26694.
4. Ma Y, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed 2016;29:1546–1552 doi: 10.1002/nbm.3609.
5. Ma Y, Chang EY, Carl M, Du J. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn Reson Med 2017;79:692–700 doi: 10.1002/mrm.26716.
6. Zhu Y, Cheng X, Ma Y, et al. Rotator cuff tendon assessment using magic-angle insensitive 3D ultrashort echo time cones magnetization transfer (UTE-Cones-MT) imaging and modeling with histological correlation. Journal of Magnetic Resonance Imaging 2018;48:160–168 doi: 10.1002/jmri.25914.
7. Ma Y, Lu X, Carl M, et al. Accurate T 1 mapping of short T 2 tissues using a three-dimensional ultrashort echo time cones actual flip angle imaging-variable repetition time (3D UTE-Cones AFI-VTR) method. Magn Reson Med 2018;80:598–608 doi: 10.1002/mrm.27066.
8. Ma YJ, Zhao W, Wan L, et al. Whole knee joint T1 values measured in vivo at 3T by combined 3D ultrashort echo time cones actual flip angle and variable flip angle methods. Magnetic Resonance in Medicine 2019;81:1634–1644 doi: 10.1002/mrm.27510.
9. Ma Y, Tadros A, Du J, Chang EY. Quantitative two-dimensional ultrashort echo time magnetization transfer (2D UTE-MT) imaging of cortical bone. Magn Reson Med 2018;79:1941–1949 doi: 10.1002/mrm.26846.
Figures