5111
On the elimination of the fat saturation effect in ultrashort echo-time imaging of Achilles tendons.1Masaryk University, Brno, Czech Republic, 2Medical University of Vienna, Vienna, Austria, 3Institute of Scientific Instruments, Czech Academy of Sciences, Brno, Czech Republic
Synopsis
Keywords: Tendon/Ligament, Skeletal, Achilles tendon, Bi-exponential . T2*
We investigated tri‑component analysis of 2D-UTE in-vivo measurements on a clinical 3T scanner and tested its ability to eliminate the effect of fat suppression on short T2* values in Achilles tendons. We found that fat-suppressed acquisition with two-component analysis provides ratios of short-T2* to long-T2* component intensities that are substantially lower (by a factor 0.95 to 0.55) than those resulting from fat-unsuppressed acquisition and tri-component fitting. Hence it seems that the combination of 2D-UTE acquisition with tri-component analysis might contribute to further improvement of T2* estimation accuracy.
PURPOSE
Bi-exponential T2* analysis of the Achilles tendon is often employed for obtaining information about its composition, aiming to increase the diagnostic reliability (1). Fat suppression is a common practice in musculoskeletal imaging scenarios as it prevents various chemical shift artifacts and enhances contrast resolution (2). However, an undesirable side effect of the typical fat suppression technique using chemical shift saturation is partial reduction of the short T2* signal, which causes alteration of the signal composition assessment (3). Different approaches mitigating such effect in ultrashort echo time (UTE) imaging were suggested, such as employment of optimized composite fat suppression RF pulses (4), fat suppression with a single-point Dixon method (5) or 3D-UTE with tri-component analysis (6). In this study, we investigated the feasibility of tri-component T2* calculation using 2D-UTE data acquisition, and its impact on T2* calculation accuracy in the Achilles tendon in-vivo.
MATERIAL AND METHODS
The experiments were performed on a 3T MRI system (Magnetom Prisma, Siemens Medical Solutions, Erlangen Germany) using a 4-channel flexible coil and a homemade holder for precise positioning. The diagram of the custom-made time-interleaved 2D-UTE pulse sequence used in this study is depicted in Fig. 1. A single sagittal and axial slice was acquired with following parameters: FOV = 170 mm, TR = 60 ms, flip angle FA=250, 64‑echoes with echo time ranging from 60 µs up to 35.2 ms, and a total scan time of 6:23 minutes. Each measurement was performed twice, with a fat saturation module turned on and off. Image reconstruction was performed off-line from raw data using algorithms and routines written in MATLAB (The MathWorks, Natick, MA, USA). Five volunteers (mean age 32 ± 4 years) with healthy Achilles tendons with no history of injury were involved in the study. The T2* values were determined from magnitude data, using the mean intensity of region-of-interest (ROI) and the following models:Two component model – was used for fat-suppressed data and is based on formula with five fitting parameters:
$$\vert{S(t_n)}\vert = A_S e^{-\frac{t_n}{T_{2S}^*}} + A_L e^{-\frac{t_n}{T_{2L}^*}} + C\qquad(1)$$
where $$$T_{2S}^*$$$ and $$$T_{2L}^*$$$ are $$$T_{2}^*$$$ values of the short and long relaxation components, respectively, $$$A_S$$$ and $$$A_L$$$ are their fractions, $$$t_n$$$ is the n-th echo time and C represents the offset caused by noise.
Three component model – was applied for fat-unsuppressed data, exploiting four additional parameters, which reflect the fat contribution to the sampled signal. This model with nine parameters in total is:
$$\vert{S(t_n)}\vert = A_S e^{-\frac{t_n}{T_{2S}^*}} + A_L e^{-\frac{t_n}{T_{2L}^*}} + A_F e^{-\frac{t_n}{T_{2F}^*}} \sum_{p} \alpha_p e^{i(2\pi(f_p + \Delta f_{wf})t_n + \Delta P_{wf})} + C\qquad(2)$$
where fat is modeled by a sum of peaks with relative amplitudes $$$\alpha_{p}$$$ and frequency shifts $$$f_{p}$$$ , the $$$A_{F}$$$ overall fat fraction and the fat relaxation time $$$T_{2F}^*$$$ . For the purpose of this study we adopted a 7-peak subcutaneous fat model as presented in (7). The two remaining parameters are the water‑fat frequency shift $$$\Delta f_{wf}$$$ and the initial water/fat phase offset $$$\Delta P_{wf}$$$. We introduced the first parameter to cope with the fact that fat is usually located outside of the selected slice and therefore its resonance frequency might be altered by field inhomogeneity. The second one $$$\Delta P_{wf}$$$ reflects the initial water/fat phase shift accumulated during slice selective excitation.
For the analyses, three ROIs in the sagittal and one in the axial slice orientation were defined manually and are shown in Fig. 2. The data fitting procedures were based on a MATLAB least‑squares curve‑fitting algorithm.
RESULTS
An example of $$$T_2^*$$$ analysis for a sagittal slice and a ROI positioned over the insertion part of the Achilles tendon is shown in Fig. 3. The ratio of the short and the long fractions, defined as:$$F_{SL} = \frac{A_S}{A_L}\qquad(3)$$
was calculated for a particular ROI of each subject. The average values obtained from the whole group are shown Fig. 4(A). Fig. 4(B) shows the relation of fraction ratios (FR) of both analyses, which we define as:
$$FR=\frac{(F_{SL})_{2-comp}}{(F_{SL})_{3-comp}}\qquad(4)$$
Finally, the results from group analysis are summarized in Tab. 1.
DISCUSSION
In this study, we analyzed the performance of T2* mapping from 2D-UTE sequences with and without fat saturation. The off-resonance oscillations resulting from fat contamination were well fitted with a tri-component model as previously suggested (6). The application of 2D UTE acquisition allowed us to achieve acceptable in‑vivo scanning time with 64 echoes, providing enough data points for robust water/fat separation. We introduced two additional fitting parameters to better describe the signal formation in a 2D experiment. The main limitation of the proposed method is perhaps that only a single slice was acquired. The fraction ratios obtained with fat suppression tend to be lower than those obtained without fat suppression; this was significant for MID, MTJ and MIDA regions (0.55, 0.61 and 0.57), but it appears less pronounced for INS regions where the FR factor of only 0.95 was obtained.CONCLUSION
The 2D UTE sequence demonstrated applicability for in-vivo measurement and could provide less biased information on $$$T_2^*$$$ composition of the Achilles tendon.Acknowledgements
This work was funded by Czech Science Foundation grant no. GA22‑10953S.References
1. Juras V, Apprich S, Szomolanyi P, Bieri O, Deligianni X, Trattnig S. Bi-exponential T2* analysis of healthy and diseased Achilles tendons: an in vivo preliminary magnetic resonance study and correlation with clinical score. Eur Radiol 2013;23:2814–2822 doi: 10.1007/s00330-013-2897-8.
2. Del Grande F, Santini F, Herzka DA, et al. Fat-Suppression Techniques for 3-T MR Imaging of the Musculoskeletal System. RadioGraphics 2014;34:217–233 doi: 10.1148/rg.341135130.
3. Carl M, Nazaran A, Bydder GM, Du J. Effects of fat saturation on short T2 quantification. Magnetic Resonance Imaging 2017;43:6–9 doi: 10.1016/j.mri.2017.06.007.
4. Ma Y-J, Jerban S, Jang H, Chang EY, Du J. Fat suppression for ultrashort echo time imaging using a novel soft-hard composite radiofrequency pulse. Magnetic Resonance in Medicine 2019;82:2178–2187 doi: 10.1002/mrm.27885.
5. Jang H, Carl M, Ma Y, et al. Fat suppression for ultrashort echo time imaging using a single-point Dixon method. NMR in Biomedicine 2019;32:e4069 doi: 10.1002/nbm.4069.
6. Lu X, Jerban S, Wan L, et al. Three-dimensional ultrashort echo time imaging with tricomponent analysis for human cortical bone. Magnetic Resonance in Medicine 2019;82:348–355 doi: 10.1002/mrm.27718.
7. Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res 2008;49:2055–2062 doi: 10.1194/jlr.D800010-JLR200.
8. Latta P, Starčuk Z, Kojan M, et al. Simple compensation method for improved half-pulse excitation profile with rephasing gradient. Magnetic Resonance in Medicine 2020;84:1796–1805 doi: 10.1002/mrm.28233.
Figures
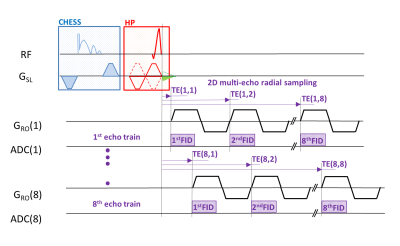
Figure 1: 2D-UTE time-interleaved, multi-echo sequence is based on the acquisition of 8 echo trains, each containing 8 echoes, i.e. of 64 echoes per one measurement. The sequence starts with optional chemical shift suppression (CHESS) module. The half-pulse variable rate–selective excitation (HP-VERSE) module exploits a triangle compensation-gradient pulse (green color) to compensate for eddy currents induced by the slice-selective gradient as described in ref. (8). Afterward, 8 half-echoes are sampled using monopolar readouts in each interleave.
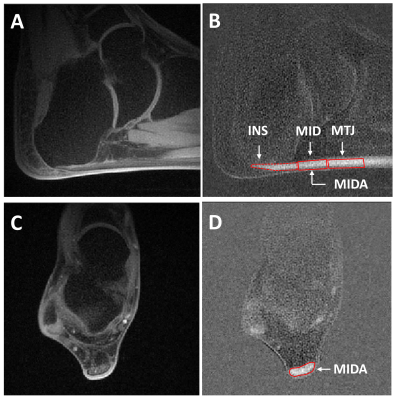
Figure 2: 2D UTE images with fat suppression acquired at TE = 60 ms for the sagittal (A) and for the axial slice orientation (C). Corresponding differential images (B) and (D) were calculated by subtracting the data acquired at TE = 60 ms and TE = 600 ms. An example of ROI placement (insertion part, INS; middle part, MID; muscle-tendon junction, MTJ) is shown for sagittal (B) and (middle part axial, MIDA) axial (D) slice orientations.
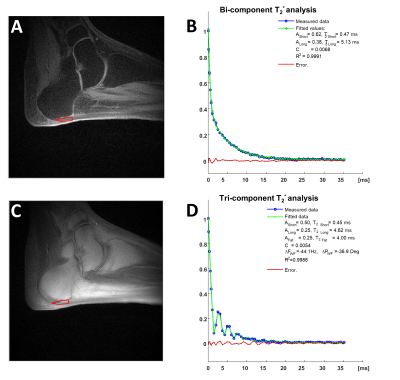
Figure 3: An example of $$$T_2^*$$$ analysis from UTE images acquired with (A) and without (C) fat suppression. The results of both types of fitting in a ROI placed over the insertion part of the Achilles tendon are shown in (B) and (C).
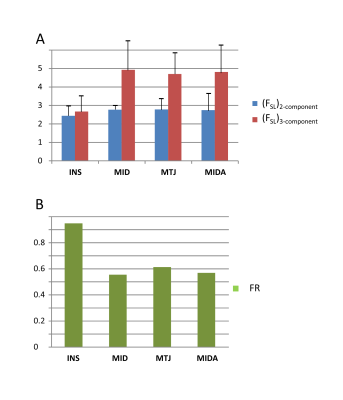
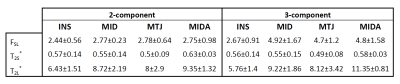
Table 1: The summary of short-to-long $$$T_{2}^{*}$$$ fraction $$$F_{SL}$$$, $$$T_{2S}^*$$$ and $$$T_{2L}^*$$$ in ms obtained from five subjects, the average values and standard deviations are shown for each calculated parameter.