5109
Adapted rosette trajectory for functional 2D lung imaging1Ulm University Hospital, Ulm, Germany
Synopsis
Keywords: New Trajectories & Spatial Encoding Methods, Lung, Function
Lung MRI is a steadily evolving field of research, especially concerning the evaluation of chronic lung diseases such as cystic fibrosis or COPD. The major limitation of lung MRI is the ultrashort T2* relaxation time of lung parenchyma, which demands ultrashort echo time sequences. Sufficient SNR values in the parenchyma are crucial for clinical evaluations and the assessment of physiological parameters. This abstract presents an adapted rosette trajectory for UTE imaging, yielding higher SNR per unit time in comparison to radial UTE sampling approaches.Introduction
Magnetic resonance imaging (MRI) of tissues or samples with ultrashort T2* relaxation times such as the lung1 requires imaging approaches with short echo times (TE). Ultrashort echo time (UTE) enables data acquisition with minimal TE by acquisition of the data from the start of the read-out gradient as opposed to only sampling when the final gradient strength is reached.To eliminate respiratory motion, breath-hold acquisitions are frequently applied to lung imaging, although highly limiting the maximum scan duration to a few seconds, especially in patients with respiratory disorder. Therefore, acquiring images at sufficient SNR during one breath-hold is crucial to obtaining high quality and quantifiable lung images. However, in conventional radial UTE the read-out gradients are rephased, followed by a constant spoiler gradient, causing an inefficient use of the rather long repetition time (TR).
In this contribution, a rosette-like k-space sampling pattern was applied, covering k-space more efficiently by additional data sampling during the required rephasing. The performance of the suggested trajectory and variants thereof is investigated in volunteers and compared to the conventional UTE approach.
Methods
The suggested approach was tested in four healthy volunteers with no reported respiratory disorders, who provided informed written content prior to the MR examination. All images were acquired during a single breath-hold each for inspiration and expiration at a 3T whole-body clinical imaging system (Ingenia 3.0T CX, Philips Healthcare, Best, The Netherlands) using one anterior coil and the posterior section of the whole-body coil. The imaging slice was centered at the bifurcation of the trachea in coronal orientation.The rosette trajectory2 was parametrized by
$$\vec{k}(t) = k_{max} \sin(\omega t) \text{e}^{it}, $$
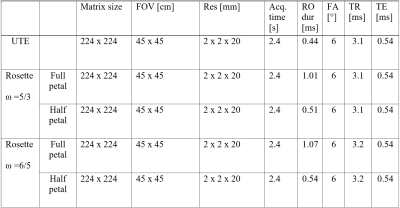
with $$$\vec{k}$$$ being the position in k-space, kmax the outmost position of the sampled k-space and ω the angular frequency. The UTE acquisition followed the traditional radial UTE center-out sampling pattern3. In both approaches an angular increment following the tiny golden angle sampling scheme (φ7 = 23.6281°)4 was used. The number of readouts for both approaches was determined to fulfill full Nyquist sampling for the radial UTE sequence. All relevant scan parameters are listed in table 1.
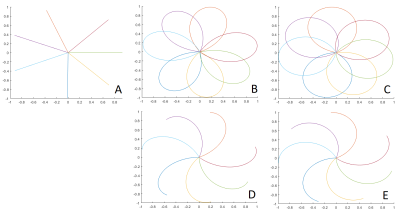
For image reconstruction, an in-house built reconstruction framework, implemented in MATLAB (The MathWorks, Natick, Massachusetts, USA) was used. The k-space density functions for the rosette trajectory were calculated using a Voronoi tessellation5. For the rosette trajectories, images were reconstructed from either complete petals or only half petals (center-out) per excitation (see figure 1). Rosette data was acquired with ω = 5/3 (figure 1 B,D), and ω = 6/5 (figure 1 C,E).
The parenchyma was segmented semi-automatically and SNR, proton fraction6 (fp) and fractional ventilation7 (FV) were calculated according to:
$$ \text{SNR} = \sqrt{\frac{2-\pi}{2}} \frac{\text{SI}_{\text{ROI}}}{\sigma_{\text{BG}}},$$
$$f_p = \frac{\text{SI}_{\text{lung}}}{\text{SI}_{\text{muscle}}} \cdot \exp\left( \frac{\text{TE}}{\text{T}_2^*}\right)\; \text{and}$$
$$\text{FV}=\frac{(\text{SI}_{\text{ex}} − \text{SI}_{\text{in}})}{\text{SI}_{\text{ex}}},$$
with SIROI being the signal intensity of a region of interest (ROI), σBG the standard deviation of background noise, SIlung the signal intensity of the lung parenchyma, SImuscle the signal intensity of the intercostal muscle. Furthermore, T2* = 0.74ms was assumed8 for lung parenchyma at 3T. SIin and SIex correspond to the signal intensity of the lung parenchyma for the inspiration and expiration state, respectively.
Results
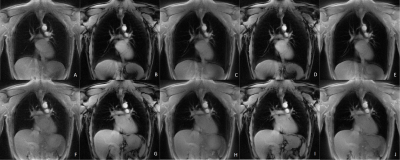
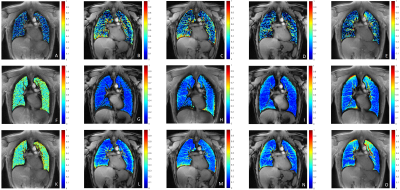
Data acquisition could be completed for all volunteers. Figure 2 shows inspiration and expiration images obtained with the radial UTE (A,F), the rosette trajectory with full petal for ω=5/3 (B,G), ω=6/5 (D,I) and the center-out half of each petal for ω=5/3 (C,H) and ω=6/5 (E,J) for a single volunteer.Derived fractional ventilation maps are provided in figure 3 for on volunteer for radial UTE (A), rosette trajectory with full petal for ω=5/3 (B), ω=6/5 (D) and center-out half of each petal for ω=5/3 (C) and ω=6/5 (E), as well as proton fraction maps for radial UTE (F,K), the rosette trajectory with full petal for ω=5/3 (G,L), ω=6/5 (I,N) and the center-out half of each petal for ω=5/3 (H,M) and ω=6/5 (J,O) for inspiration and expiration, respectively.
The SNR in the lung parenchyma resulted significantly higher (p<0.05) for inspiration and expiration, when using full petals with ω=6/5 compared to the radial UTE acquisition and (p<0.025) when using full petals with ω=5/3 compared to the radial UTE acquisition. No statistically significant difference was found between the half-petal trajectories and the radial UTE trajectory. In all acquisitions, significant differences (p<0.05) between inspiration and expiration were observed.
Fractional ventilation was significantly higher (p<0.05) for the full petal trajectories when compared with the radial UTE trajectory. No significant difference was found between the half-petal trajectories and the radial UTE acquisition.
Discussion and Conclusion
The rosette trajectories cover more k-space data without increasing repetition time or total scan duration, since each petal ends in k0, thus removing the necessity of a rephasing gradient applied prior to spoiling. The resulting oversampling of k-space leads to a significant improvement of SNR compared to a traditional radial UTE trajectory. Furthermore, the fractional ventilation is significantly higher for the adapted rosette approach, most likely due to higher SNR values in the parenchyma compared value obtained by the radial UTE images.In addition to the breath-hold images, the presented trajectory possesses great potential for gated lung imaging, since the oversampling of k-space potentially enables higher temporal resolution for image-based self-gating approaches.
Acknowledgements
The authors thank the Ulm University Center for Translational Imaging MoMAN for its support. This work was supported by the German Research Foundation funding agreement 465599659. Technical support from Philips Healthcare is gratefully acknowledged.References
1. Wild JM, Marshall H, Bock M, et al. MRI of the lung (1/3): methods. Insights Imaging. 2012;3(4):345-53.
2. Noll DC. Multishot Rosette Trajectories for Spectrally Selective MR Imaging. IEEE. 1997;16(4): 0278–0062.
3. Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology 1991;179(3):777–781.
4. Wundrak S, Paul J, Ulrici J, et al. Golden ratio sparse MRI using tiny golden angles. Magn Reson Med 2016; 75:2372-2378.
5. Rasche V, Proksa R, Sinkus R, et al. Resampling of data between arbitrary grids using convolution interpolation. IEEE Trans Med Imaging. 1999;18(5):385-92.
6. Hatabu H, Alsop DC, Listerud J et al. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur J Radiol. 1999;29:245-252.
7. Zapke M, Topf H-G, Zenker M, et al. Magnetic resonance lung function–a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir Res 2006;7:106.
8. Yu J, Xue Y, Song HK. Comparison of lung T2* during free-breathing at 1.5 T and 3.0 T with ultrashort echo time imaging. Magn Reson Med. 2011;66:248-254.
Figures