5108
DUDE: Diffusion tensor imaging with Ultra-short echo time Double Echo steady state – a proof of concept1Siemens Healthineers International AG, Zurich, Switzerland, 2Swiss Center for Musculoskeletal Imaging (SCMI), Balgrist Campus, Zurich, Switzerland, 3Advanced Clinical Imaging Technology (ACIT), Siemens Healthineers International AG, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Keywords: Data Acquisition, Pulse Sequence Design, DESS, UTE, UTE-DESS, DW-DESS, DW-UTE-DESS, ZTE
The quality of echo-planar diffusion images can suffer from severe distortions and signal dropouts, especially in regions with high susceptibility gradients and rapidly decaying (short T2*) signal. To overcome these problems, we implemented a diffusion sensitive UTE-DESS sequence. The acquisition along six distinct diffusion directions allows for fitting of a diffusion tensor. We show the feasibility of Diffusion tensor imaging with Ultra-short echo time Double Echo steady state (DUDE) at 3T and present preliminary results in the brain of a healthy volunteer.
Introduction
The quality of echo-planar diffusion images can suffer from severe distortions and signal dropouts, especially in regions with high susceptibility gradients and rapidly decaying signal due to short T2*. Among potential alternatives are diffusion-weighted steady-state free precession (SSFP) [1, 2] or double-echo steady state (DESS) sequences, with the latter having shown promising results in assessments of, e.g., knee cartilage [3, 4, 5]. To further reduce B0-inhomogeneity-induced signal loss and to image tissue with even shorter T2* times, the DESS sequence was modified to detect signal at ultra-short echo time (UTE-DESS) [6]. Compared to DESS, UTE-DESS also benefits from reduced motion sensitivity using a radial readout trajectory.In this work, we extended the UTE-DESS sequence to be diffusion sensitive along six distinct directions to allow fitting of a diffusion tensor. Preliminary results of diffusion tensor imaging (DTI) based on the UTE-DESS sequence are presented in the brain of a healthy volunteer as a proof of concept.
Methods
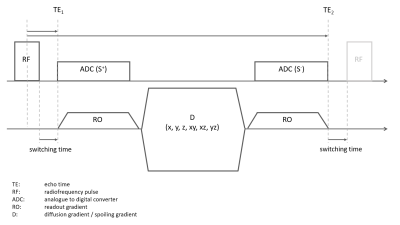
A research application sequence with a 3D radial UTE trajectory – center out (1st echo, S+) followed by a spoiling / diffusion sensitizing gradient and a center-in readout (2nd echo, S-) – was implemented and tested on a healthy volunteer at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil. A schematic diagram of the diffusion tensor imaging ultra-short echo time double echo steady state (DUDE) sequence is depicted in Figure 1. A 100 us hard block pulse (flip angle: 20°) was used for excitation. With a switching time (between RF and the first ADC and symmetrically between the ADC of the second read out and the RF pulse of the consecutive TR) of 100us, an effective echo time (TE) of 150us (dead time + ½ RF pulse duration) was achieved for the first echo. The approximate b-value for the diffusion-weighting over 1 TR period of 8 ms was b=330s/mm2 given a spoiling gradient amplitude and duration of 34 mT/m and 5ms, respectively. However, multiple stimulated echo pathways will contribute to a higher effective b-value. A total of six diffusion directions were acquired by varying the orientation of the spoiling / diffusion gradient. Acquisition of 12’000 radial spokes took 1min 36s per diffusion direction and images were reconstructed at an isotropic resolution of 1.8mm using a gradient impulse response function (GIRF) to correct for trajectory errors induced by gradient non-linearities [7].Post-processing consisted of rigid image registration to correct for motion in between diffusion directions and voxel-wise fitting of a diffusion tensor model. An averaged image across all diffusion directions with 3-fold scaled intensity was used for signal normalization during fitting instead of a commonly used non-diffusion weighted b=0 image, which cannot be acquired with the current sequence (removing the spoiling gradient completely will result in severe image artifacts).
Restults
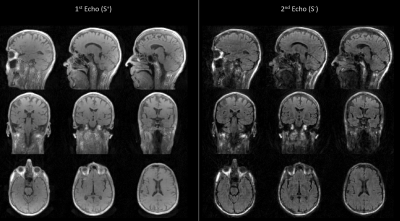
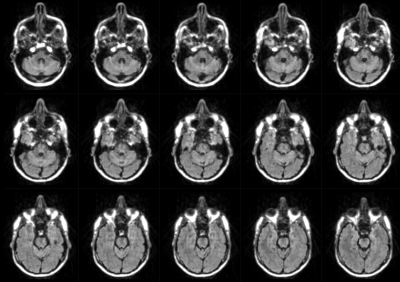
Figure 2 depicts the first and second echo images in all three orthogonal planes of the DUDE sequence with diffusion encoding applied in Y (anterior – posterior) direction.Figure 3 displays diffusion-weighted images from the second echo of the DUDE sequence with diffusion encoding along X-direction (left - right) in several axial slices acquired in the lower part of the brain.
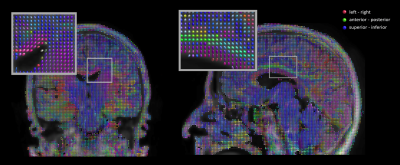
Figure 4 shows the results of a tensor fit overlayed on the image of averaged diffusion directions using the common directional encoding (red: left-right, green: anterior - posterior, blue: superior-inferior). Commonly known axonal connections such as the corpus callosum, corticospinal tract and cingulum bundle can be identified on the color-coded tensors.
Discussion
The DUDE sequence shows little to no distortions or signal dropouts, even in regions with high B0 inhomogeneity such as the lower parts of the brain (Figure 2). In other diffusion weighted sequences with e.g. EPI readouts, these regions are typically heavily affected by image distortions and signal dropouts due to air-bone interfaces such as in the sinuses and nasal cavities. A reasonable diffusion weighting could be achieved in the second echo while non-diffusion weighted UTE FID images were acquired during the first echo.In this study, the number of diffusion directions was limited to six, which does not allow for a robust fitting of the tensor model. Nevertheless, the primary eigenvalue of the tensor looks reasonable in most anatomical areas. Further evaluation with respect to scan duration, minimum signal-to-noise-ratio (SNR), resolution and number of spokes is needed. Furthermore, identical readout trajectories were used for all diffusion directions, interleaving the radial sampling pattern across diffusion directions might allow for higher SNR and less under-sampling artifacts and the potential for joint reconstruction. Additionally, sensitivity to bulk motion e.g. originating from CSF pulsation needs further investigation.
Besides imaging the brain, another interesting application of DUDE could be in muscle tissue, cartilage, ligaments or in the vicinity of metal implants.
Conclusion
We have implemented a diffusion-weighted UTE DESS sequence and tested its application in DTI in a brain of a healthy volunteer by acquiring six distinct diffusion directions.Acknowledgements
No acknowledgement found.References
1. Wu EX, Buxton RB. Effect of diffusion on the steady-state magnetisation with pulsed field gradient. J Magn Reson 1990;90:243–253.
2. Buxton RB. The diffusion sensitivity of fast steady-state free precession imaging. Magn Reson Med 1993;29:235–243.
3. Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T2 and apparent diffusion coefficient inhuman articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med2012;67:1086–1096
4. Bieri O, Ganter C, Welsch GH, Trattnig S, Mamisch TC, Scheffler K.Fast diffusion-weighted steady state free precession imaging of invivo knee cartilage. Magn Reson Med 2012;67:691–700.
5. Bieri O, Ganter C, Scheffler K. Quantitative in vivo diffusion imaging of cartilage using double echo steady-state free precession. MagnReson Med 2012;68:720–729.
6. Jang H, Ma Y, Carl M, Jerban S, Chang EY, Du J Ultrashort echo time Cones double echo steady state (UTE-Cones-DESS) for rapid morphological imaging of short T2 tissues. MagnReson Med 2021;86:881–892.
7. Vannesjo, S. J. et al. Gradient and shim pre-emphasis by inversion of a linear time-invariant system model. Magn. Reson. Med. 78, 1607–1622 (2017).
Figures