5107
Low-Rank Plus Sparse Accelerated Proton Resonance Frequency Shift and T1-mapping with a Dual-Echo 3D Spiral Ultra-Short Echo Time Sequence1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Netflix, Los Gatos, CA, United States, 3U.S. Food and Drug Administration, Silver Spring, MD, United States, 4Brigham Young University, Provo, UT, United States, 5Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
Keywords: Sparse & Low-Rank Models, Image Reconstruction, UTE, Spiral, Focused Ultrasound
Unintended heating of the skull and nearby brain tissue during MR-guided Focused Ultrasound (MRgFUS) treatment is not standardly monitored. A method for addressing this issue, which combines variable flip angle (VFA) T1 mapping and proton resonance frequency (PRF) shift thermometry based on a dual-echo 3D spiral ultra-short echo time (UTE) acquisition, was accelerated with a low-rank plus sparse model in image reconstruction and validated using retrospectively undersampled data in vitro.
Introduction
MR thermometry is used in monitoring non-invasive heating-induced therapies such as focused ultrasound surgery and laser interstitial thermal therapy1. During transcranial MR-guided Focused Ultrasound (MRgFUS) surgery, unintended heating of the skull and nearby soft tissue may occur, causing pain and damage. Monitoring of this heating is challenging because a large volume that contains different tissues should be covered within a short interval.A method using a dual-echo 3D spiral ultra-short echo time (UTE) sequence has been proposed to address this problem2. Variable flip angle (VFA) T1 mapping and the proton resonance frequency (PRF) shift method were applied to the data from the two echoes. However, the resulting temporal resolution was relatively low, and thus acceleration is needed.
Low-rank plus sparse (L+S) matrix decomposition is naturally suited for fast dynamic MRI3. Multi-frame thermometry data can be divided into the temporally related background component $$$L$$$, and the temporally independent overlay component $$$S$$$. In this work, we used the L+S model to accelerate reconstruction of retrospectively undersampled dual-echo 3D spiral UTE MRI data. A validation experiment was conducted with a bone-gel phantom.
Methods
The L+S model for reconstructing undersampled dynamic spiral data is represented by the convex optimization problem below3:$$\min \|L\|_* + \lambda \|TS\|_1 \;\textit{ s.t. }\; E(L+S)=d \textit{,}$$where $$$\|\cdot\|_*$$$ is the nuclear norm, $$$\|\cdot\|_1$$$ is the $$$l_1$$$-norm, $$$\lambda$$$ is a weighting factor, $$$T$$$ is a sparsifying operator, $$$E$$$ is a non-uniform fast Fourier transform (NUFFT) operator, and $$$d$$$ is the raw data.A phantom was made by surrounding bovine femur cortical bone with agar gel both inside and outside. A cooling experiment was carried out with ~10°C temperature change. Fully-sampled MRI data was collected on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. The sequence parameters were: TR 30ms, TE 0.05/10ms, flip angles 15°/30°, matrix size 96x96x10, resolution 1.35x1.35x10mm3, 64 spiral interleaves, RF duration 60µs. In each of 16 cycles, one scan was performed at the lower flip angle and another at the higher. The data from the first cycle was discarded.
The data was retrospectively undersampled with an acceleration factor of four. For each frame, the spiral interleaves were selected evenly, and the starting index was incremented by three in order to increase incoherence in the acquisition space that is required by the L+S model for removing noise-like aliasing artifacts3. The coil sensitivity maps were generated by applying the ESPIRiT method4 on the first frame of data.
The VFA-T1 and PRF methods were used for the two echoes respectively on both the fully-sampled and undersampled data. Comparison was made between them for each method. Aliasing artifacts were also analyzed.
Results
The reconstructed (magnitude) images of a middle slice from fully-sampled data and from undersampled data by L+S and by NUFFT are displayed in Figure 1. The root-mean-square errors (RMSE) of the L+S groups are generally smaller than those for the NUFFT groups except for the third column. The aliasing artifacts caused by undersampling were dramatically removed by the L+S method.The VFA-T1 results of the bone are shown in Figure 2. Similar noise levels are observed in both rows. Despite the small temperature change, a slight decrease of intensity is observed in both rows, which qualitatively aligns with prior calibration research5.
The PRF temperature distributions are shown in Figure 3. Minor aliasing artifacts can be observed in the temperature maps. Although increasing iterations in the L+S model could eliminate the aliasing artifacts, it does not improve thermometry accuracy significantly. The mean temperature curves from the red rectangles in Figure 3 are plotted in Figure 4. For each flip angle, the temperature calculated from undersampled data deviates less than 1°C from the temperature from fully-sampled data.
Conclusion
A study of L+S model acceleration of PRF and VFA-T1 thermometry with a dual-echo 3D spiral UTE sequence was conducted with retrospective x4 undersampling. The aliasing artifacts were significantly removed with temporally incoherent spiral interleaves. The resulting T1 maps were close to those from fully-sampled data. The PRF temperature values showed low deviation. Future work includes SNR improvement, calibration of the bone T1-temperature coefficient, and prospective studies.Acknowledgements
This research was partly supported by NIH R01 EB028773, the Focused Ultrasound Foundation, and Siemens Medical Solutions. The authors acknowledge Josef Pfeuffer, Thomas Benkert, and Berthold Kiefer of Siemens for their help with this project.
References
[1] Odéen H, Parker DL. Improved MR thermometry for laser interstitial thermotherapy. Lasers Surg Med. 2019 Mar;51(3):286-300.
[2] Chen S, Gilbo YK, Sporkin HL, Fielden SW, Allen SP, Mugler JP, Miller GW, Meyer CH. Combining Proton Resonance Frequency Shift and T1-mapping Thermometry with a 3D Spiral Ultra-Short Echo Time Sequence. Proceedings 30th Annual Meeting ISMRM, London. 2022:2151.
[3] Otazo R, Candès E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med. 2015 Mar;73(3):1125-36.
[4] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014 Mar;71(3):990-1001.
[5] Han M, Rieke V, Scott SJ, Ozhinsky E, Salgaonkar VA, Jones PD, Larson PE, Diederich CJ, Krug R. Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI. Magn Reson Med. 2015 Dec;74(6):1548-55.
Figures
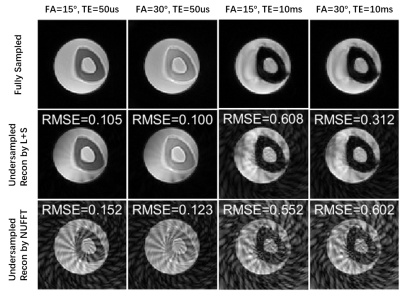
Figure 1. Images reconstructed from fully-sampled data (first row) and from undersampled data by L+S (second row), and by NUFFT (third row) are displayed for each combination of flip angle and TE. RMSEs of the undersampled data are also shown.
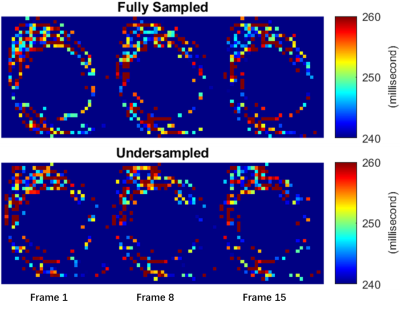
Figure 2. T1 maps of cortical bone only were generated from fully-sampled data (top row) and undersampled data by L+S (bottom row). The gel region was masked out, and the T1 maps were zoomed in to show the bone region.
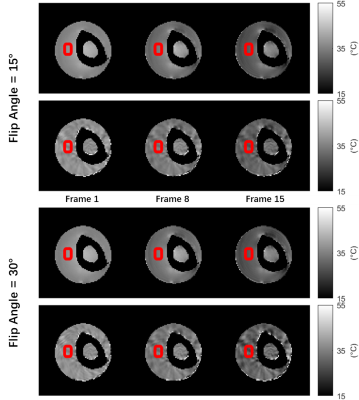
Figure 3. PRF temperature maps from fully-sampled data (first and third rows) and from undersampled data by L+S (second and fourth rows). Red rectangles are the ROIs for Figure 4.
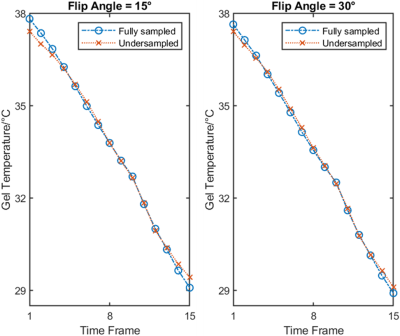
Figure 4. The spatially averaged PRF temperature curves for the ROIs in Figure 3.