5062
Relationships among brain controllability, cognition, and clinical symptoms of major depressive disorder1Department of Radiology, Huaxi MR Research Center (HMRRC), West China Hospital of Sichuan University, Chengdu, China
Synopsis
Keywords: Psychiatric Disorders, Brain, major depressive disorder
Major depressive disorder (MDD) showed both clinical symptoms and cognitive deficits. Prior studies have typically examined either symptoms or cognition correlated with brain measures, thus causing a paucity of stable brain markers that capture the full characteristics of MDD. Sparse canonical correlation analysis was used to assess the associations between two multi-dimensional clinical measurements (symptoms and cognition) and brain controllability of MDD. Average controllability of dorsal attention network (DAN) and visual network reached high associations with clinical variates in MDD, and altered controllability of DAN in patients could induce impairment of cognitive flexibility, and thus cause severe depressed mood.
INTRODUCTION
Major depressive disorder (MDD) is characterized by both clinical symptoms and cognitive deficits. Prior studies have typically examined either symptoms or cognition correlated with brain measures, thus causing a notable paucity of stable brain markers that capture the full characteristics of MDD. Brain controllability derived from newly proposed brain model integrating both metabolism (energy cost) and dynamics from a control perspective has been considered as a sensitive biomarker for characterizing brain function. Thus, identifying such a biomarker of controllability related to both symptoms and cognition may provide a promising state monitor of MDD.METHODS
Sparse canonical correlation analysis (sCCA) was used to investigate the association between brain controllability at a network level and both clinical symptoms and cognition in 99 first-episode mediation-naïve patients with MDD. sCCA conducts Pearson correlation between linear combinations of each multivariate dataset rather than focusing on correlation of each variable with other variables, thus can estimate the relationships of multivariate in one model limiting the probability of Type I error 1. To improve the reliability and reproducibility of our sCCA analysis, we combined both machine learning technique and bootstrap method to conduct sCCA model selection as in prior works 2,3. Only the models met the following robustness criteria were reported: 1) being statistically significant (PBonferroni<0.05); 2) with a median redundancy reliability score being not less than 0.8 3; and 3) with an average canonical correlation of test sets being at least half that of training sets. To improve the reliability of estimated canonical weight of features, a bootstrapping procedure (which uses random sampling with replacement) was conducted. We only regarded the features whose confidence interval of canonical weight did not cross zero as stably contributed features. After identifying the controllability pattern in relation to both clinical symptoms and cognitive functions, we further investigated the possible mediation effect of cognition on the relationship between brain controllability and clinical symptoms in patients with MDD.RESULTS
Average controllability was significantly correlated with both symptoms and cognition (rmean=0.54, PBonferroni=0.03) (Figure 1A). Average controllability of dorsal attention network (DAN) (r=0.46) and visual network (r=0.29) had the highest correlation with both symptoms and cognition (Figure 1B). Additionally, cognitive flexibility fully mediated the association between average controllability of DAN and depressed mood (indirect effect=-0.11, 95% CI [-0.18, -0.04], P=0.001) in MDD.DISCUSSION
DAN is responsible for the top-down control of attention 4, and disrupted control of attention shifts may contribute to emotional and cognitive dysfunction which has been documented in MDD 5. We quantified the controllability of brain networks by a brain control model and demonstrated the association between average controllability of DAN and both cognition and clinical symptoms, which provide direct evidence for above theoretical inference. Our mediation analysis uncovered that the relationship between average controllability of DAN and depressed mood appears to be mediated by cognitive flexibility in MDD. The findings showed that altered average controllability of DAN in patients could induce impairment of cognitive flexibility and thus cause severe depressed mood. Control of brain states transition can be manipulated with tools outside our brain 6,7, and therefore our study opens the avenue to possibly consider the controllability of DAN as an index for developing brain-machine interface interventions in alleviating depressed mood of MDD. Visual network is another brain functional network related to both clinical symptoms and cognition. Abnormal visual processing has been highlighted in patients with MDD 8, and dysfunction of visual network was correlated with depressive symptoms 9. Visual network is regulated by top-down control of DAN, and patients with MDD have shown disrupted top-down regulation of visual perception 10. Abundant literature revealed both abnormalities within and between DAN and visual network in MDD 10,11. The accumulated evidence and our findings indicated that both DAN and visual network were involved in the pathophysiology of MDD.CONCLUSION
Brain average controllability was correlated with both clinical symptoms and cognition in first-episode mediation-naïve patients with MDD. The results suggest that average controllability of DAN and visual network reached high associations with clinical variates in MDD, thus these brain features may serve as a stable biomarker to control the brain functional states transitions to be relevant to cognitions deficits and clinical symptoms of MDD. Additionally, altered average controllability of DAN in patients could induce impairment of cognitive flexibility, and thus cause severe depressed mood, which indicating that controllability of DAN may be serve as an intervation target for alleviating depressed mood through improving cognitive flexibitliy in MDD.Acknowledgements
All authors declare no biomedical financial interests or potential conflicts of interest.
References
1. Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess 2005;84(1):37-48.2. Modabbernia A, Reichenberg A, Ing A, et al. Linked patterns of biological and environmental covariation with brain structure in adolescence: a population-based longitudinal study. Mol Psychiatry 2021;26(9):4905-4918.
3. Moser DA, Doucet GE, Lee WH, et al. Multivariate Associations Among Behavioral, Clinical, and Multimodal Imaging Phenotypes in Patients With Psychosis. JAMA Psychiatry 2018;75(4):386-395.
4. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3(3):201-215.
5. Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, Williams LM. Paying attention to attention in depression. Transl Psychiatry 2019;9(1):279.
6. Barbero-Castillo A, Riefolo F, Matera C, et al. Control of Brain State Transitions with a Photoswitchable Muscarinic Agonist. Adv Sci (Weinh) 2021;8(14):e2005027.
7. Iyer KK, Hwang K, Hearne LJ, et al. Focal neural perturbations reshape low-dimensional trajectories of brain activity supporting cognitive performance. Nat Commun 2022;13(1):4.
8. Desseilles M, Balteau E, Sterpenich V, et al. Abnormal neural filtering of irrelevant visual information in depression. J Neurosci 2009;29(5):1395-1403.
9. Zhang X, Shang X, Seth I, et al. Association of Visual Health With Depressive Symptoms and Brain Imaging Phenotypes Among Middle-Aged and Older Adults. JAMA Netw Open 2022;5(10):e2235017.
10. Chen H, Liu K, Zhang B, et al. More optimal but less regulated dorsal and ventral visual networks in patients with major depressive disorder. J Psychiatr Res 2019;110:172-178.
11. Javaheripour N, Li M, Chand T, et al. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl Psychiatry 2021;11(1):511.
Figures
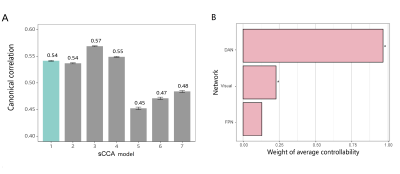
Figure 1. Sparse canonical correlation analysis (sCCA) for average controllability. Panel A shows the mean canonical correlation of sCCA models, where the model that met all three criteria was highlighted in green. Panel B shows the canonical weight of average controllability of the first sCCA model. * in panels B represent the stably contributed features whose confidence intervals did not cross zero. Abbreviations: FPN, fronto-parietal network; DAN, dorsal attention network.