5051
Diffusion Brain Imaging in Autistic Adolescents with and without Speech Impairment1University of California San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Psychiatric Disorders, Pediatric, autism, diffusion
In this study, we performed diffusion weighted imaging in subjects with Autism Spectrum Disorder and typically growing controls (TDC) to compare the differences in apparent diffusion coefficient (ADC) values between the two groups and examine their relationship to speech impairment.INTRODUCTION
Autism Spectrum Disorder (ASD) is a neurodevelopment disorder. Previous studies have demonstrated the abnormality in the structural and functional connectivity existed in several brain regions of ASD1,2,3. Speech deficits are prevalent but the pathological processes in ASD are poorly understood. In this study, we performed diffusion-weighted imaging in ASD and typically growing controls (TDC) to compare the differences in apparent diffusion coefficient (ADC) values between the two groups and examine their relationship to speech impairment.METHODS
In this study, we evaluated 35 participants with ASD (11.9±2.7 yrs; Sex: 19M/16F) and 25 TDC participants (12.3±2.7 yrs; Sex: 12M/13F). Each subject was given written informed consent to participate in this study. MR data were acquired using a 32-channel coil on a 3T GE MR750 scanner. Anatomic images included a T1-weighted sagittal MPRAGE (matrix = 256x256, FOV=25.6 cm, slice thickness = 1.0 mm), a T2 sagittal CUBE T2 (matrix = 256x256, FOV=25.6 cm, slice thickness = 1.0 mm), and 30-directional diffusion-weighted imaging (DWI) (FOV=35.0 cm, matrix = 128x128, slice thickness = 1.7-2.7mm, b=1000 s/mm2). The presence of imaging abnormality was ruled out based on T2-weighed images by a pediatric neuroradiologist. The apparent diffusion coefficient (ADC) values were calculated on a voxel-by-voxel basis using the software developed in-house4,5 and then resampled to T1-weighted images at the resolution of 1x1x1.5 cm. The segmentation of regions of interest (ROIs) was performed on T1 images using automated anatomical labeling atlas 3 (AAL3)6 by nonlinear registration. The median ADC values were calculated per ROI. Only those cerebral ROIs with a volume larger than 0.225 cm3 (150 pixels) were included in the analysis. All the participants were classified in terms of speech fluency via the NEPSY-II Inhibition Naming Time scaled score and speech accuracy via the Goldman Fristoe Test of Articulation-3rd Edition (GFTA-3) Sounds-In-Words subtest standard score. Participants who scored in the Average or above range on both measures (Naming Time >=6 and GFTA-3>=80) were classified as speech-intact. Those who were below average on either speech fluency or speech accuracy were classified as intermediate. Finally, those who scored in the below average range on both measures were classified as speech impaired. Statistical analyses were performed using R. Spearman rank correlation coefficients were calculated to examine the relationship between the ADC values and age or sex. Multivariable linear regression models were used to evaluate the differences in the ADC values between the ASD and TDC participants and their relationship to verbal abilities in the ASD.RESULTS
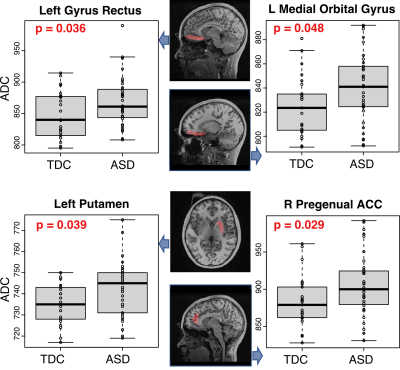
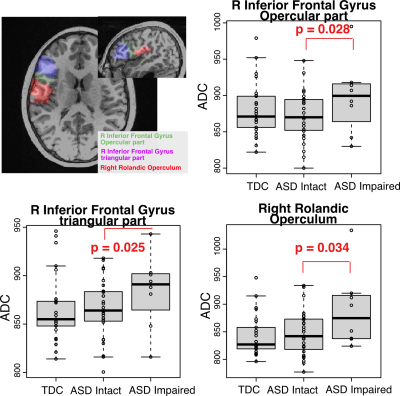
There was no statistically significant difference between ASD and TDC with respect to age or sex. In TDC, ADC values in several ROIs were age and sex dependent, such as medial orbital gyrus (left, p=0.028, age, r = 0.439; right, p = 0.006, age, r = 0.536). After adjusting for age and sex, statistically significant higher ADC values were found in the ASD in the left gyrus rectus (p=0.036), left medial orbital gyrus (p=0.048), left putamen (p=0.039), and right pregenual anterior gyrus (p=0.029) (Figure 1). Of the 35 ASD, 27 had intact speech, while 8 had intermediate to impaired speech. In ASD, when comparing those with intact speech with those with impaired speech, significant differences were within the right inferior frontal gyrus and right Rolandic operculum (Figure 2).DISCUSSION AND CONCLUSION
The present study evaluated white matter integrity in children with ASD using diffusion-weighted images. Compared to the TDC, ASD had increased ADC in the left gyrus rectus, left medial orbital gyrus, left putamen and right pregenual anterior gyrus, where functional connectivity was decreased in the ASD3. The other major finding is the ASD with impaired speech had higher ADC in the right inferior frontal gyrus and right Rolandic operculum. Of note, Broca’s area, which is responsible for processing speech and language, is located in the posterior inferior frontal gyrus. Therefore, increased ADC in this area could contribute to speech impairment in this group. Future work will use DTI tractography to examine the tract-specific impairment in ASD, as well as to evaluate their association with clinical measures, such as IQ.Acknowledgements
This study was supported by NIH R01DC019167.References
1. Sundaram SK, Kumar A, Makki MI, et al. Diffusion Tensor Imaging of Frontal Lobe in Autism Spectrum Disorder. Cereb Cortex. 2008;18(11):2569-2665.
2. Shukla DK, Keehn BBS, Lincoln AJ, et al. White Matter Compromise of Callosal and Subcortical Fiber Tracts in Children with Autism Spectrum Disorder: A Diffusion Tensor Imaging Study. 2010; 49(12): 1269-1278.
3. Xu S, Li M, Yang C, et al. Altered Functional Connectivity in Children With Low-Function Autism Spectrum Disorders. Front Neurosci. 2019; 13:806.
4. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209-119.
5. Li Y, Lupo JM, Polley MY, et al. Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro-Onc. 2011; 13(5):546-557.
6. Rolls ET, Huang CC, Lin CP, et al. Automated anatomical labelling atlas 3. NeuroImage. 2020; 206: 116189.