5046
Prediction of early response to antidepressant medication in MDD adolescents using radiomics analysis based on brain multiscale structural MRI1Department of Radiology, The Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China, 2Department of Psychiatry, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China, 3Philips Healthcare,Guangzhou China, Guangzhou, China, 4Clinical Science, Philips Healthcare, Chengdu, China, Chengdu, China
Synopsis
Keywords: Psychiatric Disorders, Psychiatric Disorders, Major depressive disorder; Antidepressant medication; Magnetic resonance imaging; Radiomics; Machine learning
Based on the radiomics analysis framework and using machine learning, this study constructed a multiscale structural MRI prediction model to predict the early response of MDD patients to antidepressant medication, and determined the radiomics features with high weight for SSRIs/SNRIs selection. The results showed that the baseline radiomics model after normalization can effectively predict the early treatment response of ADM in adolescent MDD patients, and is superior to the model based on conventional imaging indicators and unnormalized radiomics features. The AUC, accuracy, sensitivity and specificity of predicting SSRIs improvement and SNRIs improvement are 0.954, 89.2%, 87.4% and 88.5%, 0.942, 91.9%, 82.5% and 86.8%, respectively.
Background
Due to biological heterogeneity, 60%~70% patients with MDD have no response to first-line antidepressant medication (ADM). Neuroimaging biomarkers that can predict the efficacy of early response may be helpful to the personalized selection of initial antidepressant drugs. We aimed to use radiomics strategy to predict the early response to ADM in adolescents MDD by using multiscale structural brain MRI after normalization and to compare it with the prediction model based on conventional imaging indicators and unnormalized radiomics features.Materials and Methods
139 hospitalized patients with MDD were recruited for baseline brain structural MRI (3D-T1WI and DTI). After receiving SSRIs or SNRIs for 2 weeks, the subjects were divided into improved group (SSRIs improvement or SNRIs improvement) and non-improved group according to the early reduction rate of HAMD-17 score. N4 bias field correction and histogram-matching normalization were performed on the preprocessed T1WI and DTI images. Then, A.K. software (Artificial intelligence Kit) was used to extract the radiomics features of gray matter and white matter based on the voxel-based morphometry (VBM) and surfaced-based morphometry (SBM), and ComBat was used to harmonize the data. The two-level screening strategy of ANOVA and recursive feature elimination was used to reduce the dimension of radiomics features. Support vector machine (SVM) was used to integrate the multiscale sMRI of brain to construct an early efficacy prediction model. The relative importance of each feature to the prediction model was compared and the performance was evaluated by recursive feature elimination algorithm and the Leave-one-out cross-validation (LOO-CV). The ROC curve, AUC, accuracy, sensitivity and specificity based on the LOO-CV results were used to evaluate the performance of the prediction model. The Delong nonparametric test was used for AUC multiple comparisons, and the permutation test was used to evaluate the generalization ability of the model.Results
122 patients with MDD were finally enrolled, including 67 patients in the ADM improved group (31 for SSRIs improvement, 36 for SNRIs improvement) and 55 patients in the non-improved group. After 2 weeks of ADM, the HAMD17 score (reduction rate ≧ 20%) in the ADM improved group was significantly lower than that in the non-improved group (reduction rate < 20%) (P < 0.05). There was no significant difference in HAMD17 score between SSRIs improved group and SNRIs improved group (P>0.05). A total of 398 conventional image indicators including 35 features for SBM, 102 features for VBM, 261 diffusion features for DTI) and 2990 radiomics features including 667 features for SBM, 1410 features for VBM, 913 diffusion features for DTI were extracted after preprocessing. After two-level dimensionality reduction, only 8 conventional image indicators and 49 radiomics features were selected. The prediction model based on the radiomics features after normalization had the best performance. The AUC, accuracy, sensitivity and specificity of predicting ADM improvement, SSRIs improvement and SNRIs improvement are 0.889, 91.2%, 80.1% and 85.1%, 0.954, 89.2%, 87.4% and 88.5%, 0.942, 91.9%, 82.5% and 86.8%, respectively. The radiomics features that predicted the early improvement of ADM were mainly located in the anteromedial orbitofrontal cortex, superior frontal gyrus, hippocampus, cingulate gyrus, amygdala, cerebellum, corpus callosum, internal capsule and crown of superior radiation.Discussion
Personalized treatment is the long-term goal of psychiatry in clinical practice. The biomarkers for predicting the efficacy after 2 weeks determined in this study can help to select the best antidepressants, to improve the early remission rate and the long-term treatment response. The results of this radiomics framework, by quantifying the weight contribution of each image feature, confirmed the predictive ability of structural changes in gray matter and white matter in important brain regions, and can be used for the selection of antidepressants. By integrating multiscale sMRI features, the effectiveness of the prediction model is improved, and has good generalization performance, which also makes the results of the model more interpretable.In terms of prediction performance, the accuracy of the radiomics model after normalization is significantly improved compared with the prediction model based on conventional image indicators. The integration of multi-scale image features improves the prediction efficiency of features. The advantages of machine learning modeling are mainly reflected in the selection of features1-3. Predictive power may be the result of specific therapeutic drugs relieving or eliminating structural or functional pathological changes4. SBM based brain surface morphology features have a high weight in MDD diagnosis and prediction models based on conventional image indicators, while none of the indicators is selected in radiomics prediction models. In most studies, abnormal brain surface morphology of MDD has been reported as a predictor of antidepressant treatment response5. However, relevant neurophysiological studies have found that the low level of serotonin transporter in the amygdala is related to no remission 6,7, so the relationship between SBM imaging indicators and MDD drug efficacy needs to be further explored.
Conclusion
The results of this study suggest that the radiomics prediction model based on multiscale structural brain MRI after normalization at baseline could effectively predict the early treatment response of ADM in MDD patients, and the radiomics features that are predominant and discriminant for SSRIs / SNRIs selection may help for the clinical treatment and drug selection of MDD at the individual level.Acknowledgements
NOReferences
1. Leithner D, Schoder H, Haug AR, et al. Impact of ComBat harmonization on PET radiomics-based tissue classification: a dual-center PET/MR and PET/CT study. J Nucl Med. 2022, 121: 263102.
2. Saint Martin MJ, Orlhac F, Akl P, et al. A radiomics pipeline dedicated to breast MRI: validation on a multi-scanner phantom study. MAGMA. 2021, 34(3): 355-366.
3. Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006, 30(2): 436-443.
4. Korgaonkar MS, Williams LM, Song YJ, et al. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry. 2014, 205(4): 321-328.
5. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000, 10(3): 206-219.
6. Korgaonkar MS, Williams LM, Song YJ, et al. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry. 2014, 205(4): 321-328.
7. Jiang J, Zhao YJ, Hu XY, et al. Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J Psychiatry Neurosci. 2017, 42(3): 150-163.
Figures
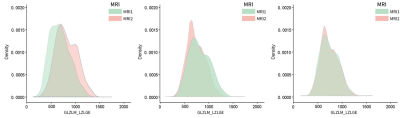
Fig. 1 Statistical distribution of LZLGE texture feature intensity extracted from 3D-T1WI images acquired by two 3.0T MRI devices A. Intensity distribution of original T1WI image; B. Intensity distribution corrected by HM normalized N4 (full mask, level 5) C. Intensity distribution corrected by HM normalized N4 and further harmonized by ComBat

Fig.2 Weights of 49 radiomics features after normalization for predicting early ADM response in MDD patients. The gray bar represents the weight value, and the darker the color is, the greater the weight is; Lighter colors mean less weight.