5034
AI-based mapping from MRI to MR thermometry for MR-guided laser interstitial thermal therapy using a conditional generative adversarial network
Saba Sadatamin1,2, Steven Robbins3, Richard Tyc3, Adam C. Waspe2,4, Lueder A. Kahrs1,5,6,7, and James M. Drake1,2,8
1Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Posluns Centre for Image Guided Innovation & Therapeutic Intervention, Hospital of Sick Children, Toronto, ON, Canada, 3Monteris Medical, Winnipeg, MB, Canada, 4Department of Medical Imaging, University of Toronto, Toronto, ON, Canada, 5Medical Computer Vision and Robotics Lab, University of Toronto, Toronto, ON, Canada, 6Department of Mathematical & Computational Sciences, University of Toronto Mississauga, Toronto, ON, Canada, 7Department of Computer Science, University of Toronto, Toronto, ON, Canada, 8Department of Surgery, University of Toronto, Toronto, ON, Canada
1Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Posluns Centre for Image Guided Innovation & Therapeutic Intervention, Hospital of Sick Children, Toronto, ON, Canada, 3Monteris Medical, Winnipeg, MB, Canada, 4Department of Medical Imaging, University of Toronto, Toronto, ON, Canada, 5Medical Computer Vision and Robotics Lab, University of Toronto, Toronto, ON, Canada, 6Department of Mathematical & Computational Sciences, University of Toronto Mississauga, Toronto, ON, Canada, 7Department of Computer Science, University of Toronto, Toronto, ON, Canada, 8Department of Surgery, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Machine Learning/Artificial Intelligence, MR-Guided Interventions, Laser Interstitial Thermal Therapy
MR-guided laser interstitial thermal therapy (MRgLITT) is a minimally-invasive treatment for brain tumors where a surgeon inserts a laser fiber along a fixed trajectory. Repositioning the laser is invasive and predicting thermal spread close to heat sinks is difficult. To address this problem, MR thermometry prediction using artificial intelligence (AI) modeling will be developed to aid the surgeon to determine whether a selected laser position is ideal before the treatment starts. AI algorithms will be trained to model the nonlinear mapping from anatomical MRI planning images to MR thermometry. A surgeon will choose a better fiber trajectory by AI model.Introduction
Epilepsy is a common neurological disorder that affects people of all ages and causes recurring, unprovoked seizures. To treat epilepsy-inducing brain tumors, open surgery, stereotactic radiation therapy, and drug therapy have been used so far1-3. However, the tumor size, location (an open surgery method is a high risk for difficult-to-access tumors), and type (e.g., drug-resistant tumors) will make the treatment challenging. Magnetic resonance-guided laser interstitial thermal therapy (MRgLITT) has demonstrated that it is one of the most effective minimally invasive techniques for treating challenging brain tumors4.MRgLITT uses a laser fiber with a diffusing or side-firing aperture to deposit light energy to ablate the tissue by heat5. LITT needs a tiny cranial hole to insert the small laser diode implanted along a straight trajectory using stereotactic techniques. If planned correctly, it does not considerably affect the surrounding healthy tissues. LITT components are magnetic resonance compatible. As a result, LITT can be monitored continuously by MRI during the surgery with real-time MR thermometry images. MR thermometry is the thermal image constructed from the changes in MRI phase images using the proton resonance frequency shift method. In addition, the surgeon can confirm the treatment area of the laser by MRI planning images precisely4,6.
The technology has some shortcomings from the surgeon’s point of view: first, the thermal spread can be lower than what the surgeon expected to ablate the tumor close to heat sinks like ventricles and major blood vessels. Second, the surgeon inserts the laser fiber via the cranial hole as the initial step of the surgery, making it difficult to reposition the probes, as this may need additional holes and trajectory if a new laser insertion is required. Hence, the surgeon must select a treatment area as accurately as possible.
For a better target selection, the surgeon can greatly benefit from a preoperative planning tool that could predict the therapy outcomes before it starts. This work aims to find a mapping from anatomical MRI to MR thermometry images as a data-driven planning tool. By having the patients’ anatomical MRI, the surgeon will access the AI-based heat propagation distribution in predicted thermometry images to better choose the ideal laser position. This approach will reduce the number of treatments for each patient and improve the treatment outcome.
Methods
Artificial intelligence algorithms are used to model the nonlinear mapping from anatomical MRI planning images to MR thermometry images. An existing dataset of epilepsy patients’ undergoing MRgLITT surgery, was used in this study. The dataset consists of each patient’s 3D anatomical T1-weighted MRI and the MR thermometry images, acquired over three parallel image slices during the surgery (Figure 1). The noisy pixels are detected due to the low signal-to-noise ratio in thermometry calculation in some parts of the brain like bones. A conditional generative adversarial network (cGAN)7 model was trained as an AI model (Figure 2). All anatomical MRI and MR thermometry images are cropped by the center of the treatment area to avoid non-useful, computationally expensive information. In this study one slice of anatomical MRI (2D) is used that corresponded to the center slice of MR thermometry (2D). Two image domains for each patient are aligned to keep the spatially related information. A dataset of 234 patients are used to train the cGAN and 53 patient sets are used to test the network. The evaluation metric to compare the network-generated thermometry image and the real thermometry image (came from previous MRgLITT surgeries) is the sum of squared differences (SSD).Results
The developed AI-based mapping is able to predict the heat propagation distribution for new thermometry images prospective to applying the therapy. The heat propagation distribution (Figure 3) generated by the AI model, is qualitatively close to the real thermometry image as it has a light-yellow spot, corresponding to elevated temperatures, over the baseline temperature in green. Also, the SSD metric is decreasing through the training process, meaning the generated images become more similar to real ones as training iterates.Discussion
This study demonstrated that using a cGAN as an AI-model to map anatomical MRI planning images to MR thermometry images is feasible. The data-driven planning tool may enhance existing MR monitoring tools of MRgLITT to enable a surgeon to select different laser locations on the system and see the model thermometry results belonging to each potential laser location. The surgeon will be able to choose the optimal laser location by inspecting the various MRgLITT outcomes, increasing the probability of a successful treatment.Conclusion
AI-based MRgLITT planning tools will increase the probability of successfully treating brain tumors and will increase treatment efficacy, while minimizing the injury of the other brain regions by decreasing the number of repeating surgeries or laser replacements during surgery. The surgeon will access predicted thermometry images before starting the surgery to insert the laser fiber, so they can better choose the treatment area and drill the skull in a more optimized place. By adding anatomical MRI segmentation information in the next steps and using more physical patient information, we will have additional information to predict MR thermometry outcomes, providing a more optimized system to improve MRgLITT brain tumor treatments.Acknowledgements
We acknowledge funding support from the INOVAIT Network and the Natural Sciences and Engineering Research Council (NSERC).
We also thank Dr. George Ibrahim and Dr. Robert Weersink for their insight on this project and Emmanuel Olaniyonu and Mohammad Elsayyed from Monteris Medical.
References
- Karim Mithani, Clemens Neudorfer, Alexandre Boutet, Jurgen Germann, Gavin JB Elias, Alexander G Weil, Elizabeth Donner, Suneil Kalia, Andres M Lozano, James M Drake, et al. Surgical targeting of large hypothalamic hamartomas and seizure-freedom following mr-guided laser interstitial thermal therapy. Epilepsy & Behavior, 116:107774, 2021.
- Stafstrom, C. E., & Carmant, L. (2015). Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harbor perspectives in medicine, 5(6), a022426.
- England, M. J., Liverman, C. T., Schultz, A. M., & Strawbridge, L. M. (2012). Summary: A Reprint from Epilepsy Across the Spectrum: Promoting Health and Understanding1. Epilepsy currents, 12(6), 245.
- Alessandro Consales, Erica Cognolato, Mattia Pacetti, Maria Margherita Mancardi, Domenico Tortora, Giuseppe Di Perna, Gianluca Piatelli, and Lino Nobili. Magnetic resonance-guided laser interstitial thermal therapy (mr-glitt) in pediatric epilepsy surgery: State of the art and presentation of giannina gaslini children’s hospital (genoa, italy) series. Frontiers in Neurology, 12, 2021.
- Tovar-Spinoza, Z., Carter, D., Ferrone, D., Eksioglu, Y., & Huckins, S. (2013). The use of MRI-guided laser-induced thermal ablation for epilepsy. Child's Nervous System, 29(11), 2089-2094.
- Christian Hoppe and Christoph Helmstaedter. Laser interstitial thermotherapy (litt) in pediatric epilepsy surgery. Seizure, 77:69–75, 2020.
- Phillip Isola, Jun-Yan Zhu, Tinghui Zhou, and Alexei A Efros. Image-to-image translation with conditional adversarial networks. In Proceedings of the IEEE conference on computer vision and pattern recognition, pages 1125–1134, 2017, Clinical: MR-Guided Interventions.
Figures
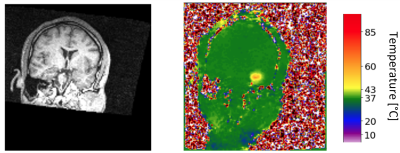
Figure 1: Two
image domains in the MRgLITT dataset for which we look for the nonlinear mapping.
Left: an anatomical MRI. Right: an MR thermometry image with color scaling.
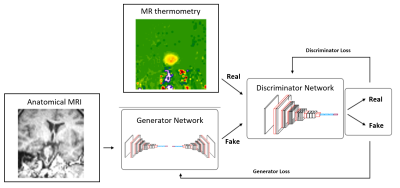
Figure
2: Conditional
GAN block diagram as an MRgLITT planning tool which is trained with anatomical
MRI and MR thermometry images cropped by the laser tip as a center– the cGAN
model consists of two independent networks, which help each other to improve
their tasks (The generator will be improved by producing more real-like
thermometry images and the discriminator by recognizing the generated
thermometry image from real ones).
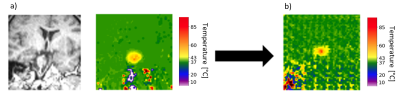
Figure 3: The cGAN input and output in the study: a) an
anatomical MRI as a generator input at left and the real thermometry data as a discriminator
input at right. b) a generated thermometry image as an output.
DOI: https://doi.org/10.58530/2023/5034