5025
Prospective motion correction improves gSlider accelerated diffusion imaging1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Max Planck School of Cognition, Leipzig, Germany, 3A.A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 4Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Motion Correction, DTI
High resolution diffusion weighted imaging (DWI) is required to map cortical fibres and short association fibres in superficial white matter. The recently developed gSlider sequence allows high-resolution whole-brain DWI but is prone to motion-induced artefacts due to the long volume acquisition. We combined gSlider with prospective motion correction (PMC) using optical tracking to acquire high angular, high spatial resolution DWI. In three healthy participants, we demonstrated that PMC led to a reduction of motion artifacts, an increase of temporal SNR of around 15% and better estimates of fibre characteristics in the cortex and superficial white matter.Introduction
Intracortical fibres and cortico-cortical short association fibres running in the superficial white matter (SWM) constitute the majority of cortico-cortical connections in the human brain1. Despite their paramount importance, these two types of fibres are not included in current in vivo human connectomes, since they are not accurately captured by conventional diffusion weighted imaging (DWI), mainly due to insufficient spatial resolution2,3. High resolution (sub- to one-millimeter isotropic) whole-brain DWI is required to comprehensively map cortical fibres and short SWM association fibres. Large volume coverage, sufficient signal to noise ratio (SNR) and reasonable acquisition times can be preserved with the use of the gSlider-SMS method4-6, which combines slice selective excitation and 3D encoding. However, gSlider acquisitions are prone to image artifacts arising from subject motion during the 10-20s it takes to acquire the differently-encoded versions of each slice that are combined for the final image. Prospective motion correction (PMC) with external optical tracking was previously shown to efficiently reduce motion artifacts in DWI7,8. Herein we incorporated PMC into gSlider and quantitatively assessed its effect on image quality both in the presence and absence of intentional motion.Methods
High angular, high spatial resolution diffusion weighted imaging was carried out on three healthy participants (2f, mean age 30±3 years) on a 3T Connectom scanner (Siemens Healthcare, Erlangen, Germany) equipped with a custom 64-channel RF receive head coil9. Participants wore a bespoke dental mouthpiece on the front teeth with an attached tracking marker and were requested not to move. An optical tracking system (Kineticor, CA, USA) installed in the bore of the scanner monitored marker motion at 85 Hz. After four-point moving-average filtering, the poses were used to dynamically update the field of view immediately prior to each RF excitation10.The simultaneous multi-slice axial DWI acquisition (gSlider×MB = 5×2) was performed using volume TR 2s, echo time (TE) 62ms, matrix size 220 x 220, isotropic in-plane resolution 1mm, GRAPPA 3, Partial Fourier 6/8, 30 slices, nominal slice thickness 5mm, two interleaved shells (b=800,1800), 60 dirs per shell, 13 b=0 images and total acquisition time of 28 min. A pair of standard and reversed (PA) phase encoding images were acquired for susceptibility-induced-distortion correction. The entire acquisition was repeated with PMC enabled and disabled. Temporal SNR was calculated across 13 b=0 images and compared for gSlider acquisitions with and without PMC.
To investigate the ability of PMC to correct artifacts from motion with larger amplitudes an additional experiment with voluntary motion was performed. The participants were asked to perform low amplitude slow voluntary yaw (left to right) head movements while recording further (one b=0, two b=800) gSlider volumes with PMC enabled and disabled.
Each set of five differently-encoded gSlider volumes were combined to obtain volumes with 1mm slices (i.e. effective acquisition time 10s) using a custom Matlab script4 and further processed using MRtrix311. High resolution gSlider volumes were denoised, eddy-current-, motion- and distortion corrected. Diffusion tensor fitting and multi-tissue constrained spherical decomposition were performed.
Results
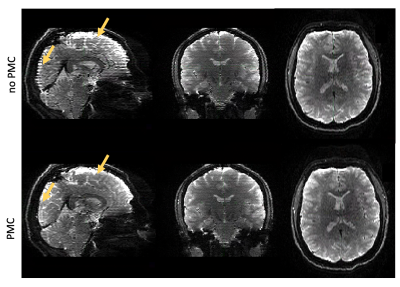
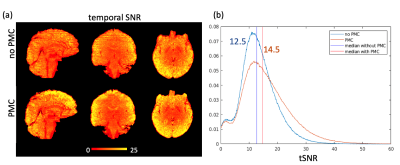
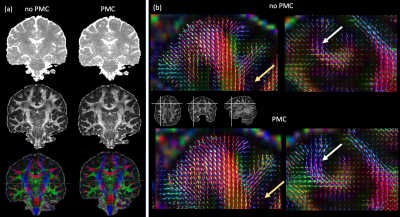
Participant motion was similar across with- and without-PMC scans, consisting mostly of a slow drift of the order of the voxel size. Motion resulted in strong variation of slice intensity across slices, with drop-outs for outer slices within 5-slice gSlider blocks. Such artifacts were especially apparent under the intentional motion condition and were suppressed when PMC was used (Fig.1). PMC reduced volume-to-volume variation of image intensity, resulting in a temporal SNR improvement of about 15% when averaged across the brain (Fig.2). With PMC correction the tSNR maps resemble the sensitivity profile of the 64ch coil, indicating efficient correction of motion and respiration induced head motion. PMC resulted in better estimates of fibre orientation distributions (FODs) in the cortex and in the superficial white matter (Fig.3).Discussion and Conclusions
We demonstrated significant reduction of motion artifacts, enhanced temporal SNR and improved mapping of cortical and SWM fibre orientation distributions for high-resolution gSlider DWI combined with PMC. As implemented, not all consequences of motion are prospectively corrected, for example movement through the receive sensitivity field, or motion during the diffusion encoding that may lead to dropouts. However, PMC – as compared with retrospective techniques – corrects slice alignment and reduces spin history effects. Furthermore, it offers a much higher temporal resolution than image based corrections. The presented method has great potential to enable submillimetre DWI in studies on large population cohorts with less compliant and less experienced participants.Acknowledgements
We thank Kawin Setsompop, Thomas Witzel and the Athinoula A. Martinos Center for Biomedical Imaging at MGH for the gSlider sequence. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905; from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project no. 347592254 (WE 5046/4-2 and KI 1337/2-2); from the Federal Ministry of Education and Research (BMBF) under support code 01ED2210; and from the National Institutes of Health under grant numbers P41EB030006 and U01EB026996. The Max Planck School of Cognition is supported by the Federal Ministry of Education and Research (BMBF) and the Max Planck Society (MPG).References
[1] Schüz, A., Braitenberg, V. The human cortical white matter: quantitative aspects of cortico-cortical long-range connectivity, In: Schüz A, Miller R, editors, Cortical Areas: Unity and Diversity, 1st ed, London and New York: Taylor & Francis. 2002; 377-385.
[2] Attar FM, Kirilina E, Haenelt D et al. Mapping Short Association Fibers in the Early Cortical Visual Processing Stream Using In Vivo Diffusion Tractography. Cereb. Cortex. 2020; 30(8):4496-4514.
[3] Schilling KG, Nath V, Hansen C et al. Limits to anatomical accuracy of diffusion tractography using modern approaches. Neuroimage. 2019; 185:1-11.
[4] Setsompop K, Fan Q, Stockmann J et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn Reson Med. 2018; 79(1):141-151.
[5] Wang F, Bilgic B, Dong Z et al. Motion-robust sub-millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magn Reson Med. 2018; 80(5):1891-1906.
[6] Liao C, Bilgic B, Tian Q et al. Distortion-free, high-isotropic-resolution diffusion MRI with gSlider BUDA-EPI and multicoil dynamic B0 shimming. Magn Reson Med. 2021; 86:791-803.
[7] Herbst M, Poser BA, Singh A et al. Motion Correction for Diffusion Weighted SMS Imaging. Magn Reson Imaging. 2017; 38:33-38.
[8] Kaso A and Ernst T. Motion-Insensitive Diffusion Imaging of the Brain using Optical Tracking and Dynamic Sequence Updates. Magn Reson Med. 2021; 86(2):926-934.
[9] Mahmutovic M, Scholz A, Kutscha N et al. A 64-Channel Brain Array Coil with an Integrated 16-Channel Field Monitoring System for 3T MRI. Proc Int Soc Magn Reson Med. 2021; 29:0623.
[10] Vaculčiaková L, Podranski K, Edwards LJ et al. Combining navigator and optical prospective motion correction for high-quality 500 μm resolution quantitative multi-parameter mapping at 7T. Magn Reson Med. 2022; 88(2):787-801.
[11] Tournier JD, Smith RE, Raffelt D et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019; 202:116-37.
Figures