5024
Single Shot Diffusion Trace Spectroscopic Imaging using Radial Echo Planar Trajectories1Radiological Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine Interdepartmental Graduate Program, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Spectroscopy, Spectroscopic Imaging
We present the first demonstration of single shot diffusion trace spectroscopic imaging using radial echo planar k-space trajectories on a clinical 3T scanner. Conventional DW-MRS requires three separate acquisitions to compute the trace apparent diffusion coefficient (ADC), whereas the single shot technique generates a trace-weighted signal in one measurement, although this has so far only been applied in NMR and to a limited extent in DW-MRI. Our preliminary results indicate good agreement with expected trace ADC values both in phantom and in healthy brain, showing a promising approach for determining the orientation-independent trace ADC value with non-Cartesian diffusion-weighted spectroscopic imaging.Introduction
Diffusion-weighted magnetic resonance spectroscopy measures the diffusion properties of metabolites at the intracellular level, in contrast to DW-MRI. Several studies have reported diffusion-weighted spectroscopic imaging (DW-MRSI) using phase encoding alone1 or echo planar k-space trajectories (EPSI)2,3. Single voxel DW-MRS approaches require shot-to-shot phase and frequency corrections to accurately determine the apparent diffusion coefficient (ADC)4,5. Previous reports using Cartesian EPSI have implemented a separate navigator echo to monitor the signal for retrospective corrections6,7. Radial trajectories (REPSI) allow for self-navigation and motion robustness8,9 due to their repeated traversal of the central k-space. The trace ADC provides an orientation-independent quantity that avoids measurement bias from diffusion anisotropy. Conventional DW-MRSI require at least three separate measurements to compute the trace ADC. However, the single-shot technique measures a trace-weighted signal in one measurement, although it has only been applied in NMR10,11,12 and to a limited extent in DW-MRI13,14. In this study, we show preliminary results of single shot trace DW-REPSI at 3T in both phantom and in vivo.Methods
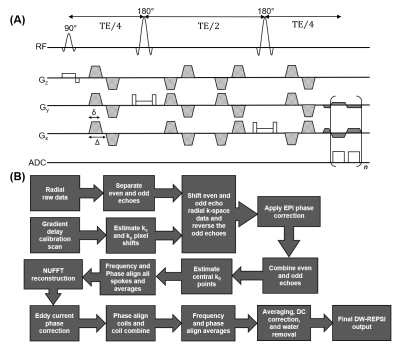
The DW-REPSI sequence applied volumetric excitation with four pairs of bipolar diffusion gradients along all axes interleaved with the slice-selective RF pulses (Figure 1). This particular configuration eliminates the bias due to cross terms between any background and diffusion gradients, and generates a diffusion trace-weighted signal in a single measurement11. A symmetric bipolar gradient readout was used to acquire kr-t data with spectral width of 1190Hz and 512 time points. The field-of-view was 32×32 cm2 with matrix size=32×32. The volume-of-interest (VOI) was 10×10×1.5 cm3 and 12×8×2 cm3, with voxel volumes of 1×1×1.5 cm3 and 1×1×2 cm3 for the phantom and in vivo data, respectively. The diffusion gradient had 1.5ms ramp time and 7.5ms total lobe duration, with 4.5ms between the bipolar pair, giving a diffusion time of 10.2ms.Gradient delay15 and EPI phase corrections16 were applied before extracting the central radial k-space point (k0) of each spoke for all time points to build a navigator signal, which was then used to phase and frequency align each average and spoke before NUFFT-based reconstruction. Water reference data for both phantom and in vivo experiments were acquired with 1 and 2 averages for low and high b-values, respectively, and were used for eddy current phase correction, coil combination, and water ADC estimation.
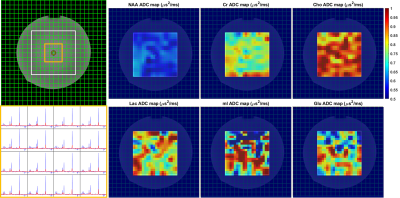
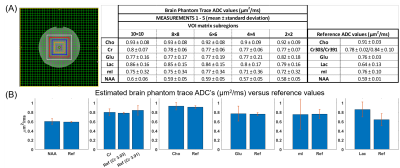
Five data sets were acquired in a phantom containing Lac, NAA, Glu, Cr, Cho, and mI. Two b-values were measured (3 and 1,411 s/mm2), using TR=2.2s, TE=150ms, 34 spokes with golden-angle distribution, and 4 and 8 averages for the low and high b-values, respectively. The mean phantom ADC values were determined in 5 regions within the 10×10 VOI matrix to determine the homogeneity of ADC values.
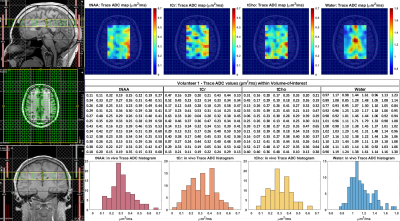
In vivo brain data from four healthy volunteers were measured using TR=2.2s, TE=142ms and two b-values – a low value ranging from 3-72 and a higher one between 1,000 – 1,231 s/mm2, corresponding to gradient amplitudes of 0-15 and 54.5-60 mT/m, respectively. For in vivo data, the maximum b-value was reduced to obtain sufficient SNR due to the long TE and greater motion-related signal losses. Also, 31 spokes with a uniform angular distribution were acquired with 6 and 12 averages for the low and high b-values, respectively, to limit the total scan time to 24 minutes. All data was quantified using LC Model17, and peak volumes with CRLB’s ≤ 30% considered for ADC calculation.
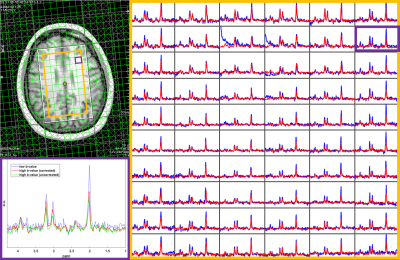
Results
The trace ADC values computed from single shot trace DW-REPSI phantom acquisitions agree well with reference values18 (Figure 3). Trace ADC maps of all six metabolites (Figure 2) show fairly homogenous values for NAA, Cr, and Cho across the VOI, although there are small fluctuations which can be attributed to B0 and B1 inhomogeneity and chemical shift displacement. The trace ADC values for mI, Glu, and Lac in the phantom have larger standard deviations, although the mean values for mI and Glu are within range of the reference values. Lactate ADC’s are overestimated by approximately 31%. Due to the long TE, only total Cr, total NAA, and total Cho were able to be reliably estimated in the in vivo data. Figure 4 shows trace ADC maps and voxel-by-voxel values of these three metabolites and water in one healthy volunteer. Because of the short diffusion time of 10.2 ms, the measured trace ADC’s are slightly higher than those determined by using diffusion-weighted MRS sequences with longer diffusion times5,19,20,21.Discussion and Conclusion
The main limitations of single-shot DW-REPSI are: (1) the long TE necessary for interleaving diffusion gradients with the localization pulses, limiting the number of detectable metabolites (i.e., NAA, Cr, & Cho), and (2) the large gradient amplitudes necessary to achieve sufficient diffusion-weighting but which induce stronger eddy current effects and greater hardware demands. The main advantage is the reduction of measurement time for trace ADC estimation, compared to other sequences that require separate measurements along three orthogonal diffusion directions. Preliminary results show good agreement in the estimated phantom ADC values, while the in vivo trace ADC’s are generally within the expected range although the values tend to be slightly higher than previously reported due to the short diffusion time of 10.2 ms which had not yet been shown for DW-MRSI in humans19,20.Acknowledgements
The authors are very grateful to Dr. Julien Valette for providing valuable insights on the single shot diffusion trace pulse sequence and on the effect of short diffusion times on in vivo ADC measurements. The authors also recognize and thank Dr. Itamar Ronen and Dr. Francesca Branzoli for their earlier support in our group’s initial investigations into single voxel diffusion-weighted spectroscopy.References
1. Ercan AE, Techawiboonwong A, Versluis MJ, Webb AG. Diffusion-Weighted Chemical Shift Imaging of Human Brain Metabolites at 7T. Magn Reson Med. 2015; 73(6):2053-2061.
2. Bito Y, Hirata K, Ebisu T, et al. Diffusion-weighted Line-scan Echo-planar Spectroscopic Imaging Technique to Reduce Motion Artifacts in Metabolite Diffusion Imaging. Magn Reson Med Sci. 2015; 14(1): 43-50.
3. Fotso K, Dager SR, Landow A, et al. Diffusion Tensor Spectroscopic Imaging of the Human Brain in Children and Adults. Magn Reson Med. 2017; 78(4):1246-1256.
4. Ellegood J, Hanstock CC, Beaulieu C. Trace Apparent Diffusion Coefficients of Metabolites in Human Brain Using Diffusion Weighted Magnetic Resonance Spectroscopy. Magn Reson Med. 2005; 53(5):1025-1032.
5. Deelchand DK, Auerback EJ, Marjanska M. Apparent Diffusion Coefficients of the Five Major Metabolites Measured in the Human Brain In Vivo at 3T. Magn Reson Med. 2018; 79(6):2896-2901.
6. Posse S, Cuenod CA, Le Bihan D. Human Brain: Proton Diffusion MR Spectroscopy. Radiology. 1993; 188(3):719-725.
7. Posse S, Cuenod CA, Le Bihan D. Motion Artifact Compensation in 1H Spectroscopic Imaging by Signal Tracking. Journal of Magnetic Resonance, Series B. 1993; 102:222-227.
8. Boer VO, Ronen I, Pedersen JO, Petersen ET, Lundell H. Metabolite diffusion weighted imaging with golden angle radial echo planar spectroscopic imaging. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. p. 3616.
9. Kim D, Adalsteinsson E, Spielman DM. Spiral Readout Gradients for the Reduction of Motion Artifacts in Chemical Shift Imaging. Magn Reson Med. 2004; 51(3):458-463.
10. Mori S, van Zijl PCM. Diffusion Weighting by the Trace of the Diffusion Tensor within a Single Scan. Magn Reson Med. 1995; 33(1):41-52.
11. de Graaf R, Braun KPJ, Nicolay K. Single Shot Diffusion Trace 1H NMR Spectroscopy. Magn Reson Med. 2001; 45(5):741-748.
12. Valette J, Giraudeau C, Marchadour C, et al. A New Sequence for Single-Shot Diffusion-Weighted NMR Spectroscopy by the Trace of the Diffusion Tensor. Magn Reson Med. 2012; 68(6):1705-1712.
13. Chun T, Ulug, AM, van Zigl PCM. Single-Shot Diffusion-Weighted Trace Imaging on a Clinical Scanner. Magn Reson Med. 1998; 40(4):622-628.
14. Wong EC, Cox RW, Song AW. Optimized Isotropic Diffusion Weighting. Magn Reson Med. 1995; 34(2):139-143.
15. Block K, Uecker M. Simple method for adaptive gradient delay compensation in radial MRI. In Proceedings of the 19th Annual Meeting of ISMRM, Quebec, Canada, 2011. p. 2816.
16. Hanson LG, Schaumburg K, Paulson OB. Reconstruction Strategy for Echo Planar Spectroscopy and Its Application to Partially Undersampled Imaging. Magn Reson Med. 2000; 44(3):412-417.
17. Provencher SW. Estimation of metabolite concentrations form localized in vivo proton NMR spectra. Magn Reson Med. 1993; 30(6):672-679.
18. Landheer K, Schulte R, Geraghty B, et al. Diffusion-weighted J-resolved Spectroscopy. Magn Reson Med. 2017; 78(4):1235-1245.
19. Marchadour C, Brouillet E, Hantraye P, Lebon V, Valette J. Anomalous diffusion of brain metabolites evidenced by diffusion-weighted magnetic resonance spectroscopy in vivo. Journal of Cerebral Blood Flow & Metabolism. 2012; 32(12):2153-2160.
20. Valette J, Ligneul C, Marchadour C, Najac C, Palombo M. Brain Metabolite diffusion form ultra-short to ultra-long time scales: what do we learn, where should we go? Frontiers in Neuroscience. 2018; 12:2.
21. Ligneul C, Valette J. Probing metabolite diffusion at ultra-short time scales in the mouse brain using optimized oscillating gradients and “short”-echo-time diffusion-weighted MRS. NMR in Biomedicine. 2017; 30(1):e3671.
Figures