5012
A novel streamline representation to reduce redundancy in tractography1Department of Computer Science, University of Verona, Verona, Italy, 2Department of Computer Science, University of Sherbrooke, Sherbrooke, QC, Canada, 3Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 4Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Genoa, Italy
Synopsis
Keywords: Tractography & Fibre Modelling, Tractography & Fibre Modelling
Diffusion MRI tractography allows one to characterize brain connectivity in vivo, and it is common practice to reconstruct millions of streamlines and filter them a posteriori. However, redundancy among streamlines leads to collinearity in the linear operators used by existing filtering algorithms. To solve this problem, we propose a novel streamline representation which uses a combination of clustering and spatial blur to reduce redundancy. This representation is as accurate as state-of-the-art filtering methods and more robust to noise/perturbations in the input, but requires only ≈5% of the input streamlines thus decreasing both storage requirements and computational complexity.Introduction
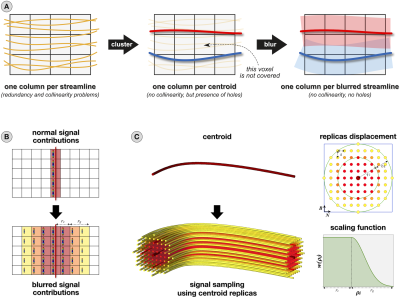
Diffusion MRI tractography is extensively used to investigate in vivo the white matter (WM) connections of the brain. A question that both beginner and expert users always must face is “How many streamlines to generate?”. To reconstruct all real pathways, it is common practice to create several millions of streamlines, even though this approach can introduce many false positives1. These tracts can be removed using microstructure informed tractography methods2, many of which (SIFT3/24, COMMIT5,6/27/2tree8, LiFE9) are based on linear formulations where each column of the linear operator typically contains the signal contributions (according to a given forward model) of a single streamline. In these circumstances, there is a critical risk, overlooked until now, when using tractograms with high cardinality: streamlines that follow very similar trajectories are stored as almost identical columns, generating collinearity in the linear operator and making it difficult to find the correct solution. To alleviate this problem, we introduce the “blurred streamlines” representation which reduces redundancy by combining streamline clustering and spatial blur of the corresponding signal contributions (Fig.1-A).Methods
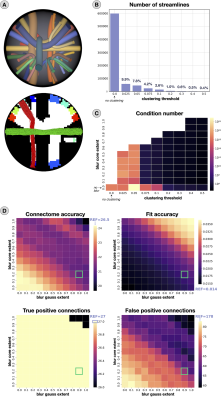
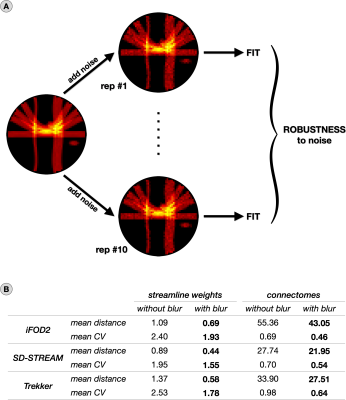
Given an input tractogram, we first pre-filter streamlines incompatible with the acquired signal and geometrical outliers. Then, we cluster the remaining streamlines bundle-by-bundle using QuickBundlesX10 and we keep only the centroids. For each centroid $$$C$$$, we spatially blur its signal contributions (according to the chosen forward model) up to a given spatial extent ($$$r=r_c+r_g$$$; in mm) to avoid uncovered WM voxels (Fig.1-B). The sampling is done empirically (Fig.1-C): we create $$$M$$$ replicas of $$$C$$$ displacing them in a Cartesian grid around $$$C$$$ itself and scaling their signal contributions as function of their distance from $$$C$$$. The replicas at distance $$$\rho_i\leq r_c$$$ contribute as much as $$$C$$$ ($$$w(\rho_i)=1$$$), whereas those at distance $$$r_c<\rho_i\leq r$$$ are scaled by the Gaussian function $$$w(\rho_i)=\exp(-0.5(r-r_c)^2/\sigma^2)$$$. Consequently, the total contribution of $$$C$$$ is computed as $$$\sum_{i=1}^Mw(\rho_i)S(C,\rho_i,\theta_i)$$$, where $$$S(C,\rho_i,\theta_i)$$$ is the signal contribution of the replica of $$$C$$$ at coordinates $$$(\rho_i,\theta_i)$$$, and will form a single column in the linear operator. Since we implemented this idea for COMMIT5,6, we name it COMMITblur.We quantitatively evaluated COMMITblur using the ISBI2013 phantom (Fig.2-A) and three tractography algorithms: iFOD211, SD-STREAM12 and Trekker13. Firstly, we investigated the condition number of the linear operator to quantify the redundancy reduction at different clustering thresholds. For the best threshold, we varied the blur extent to match COMMIT performances and we assessed the accuracy through number of true positive (TP) and false positive (FP) connections, L1 distance from the ground-truth connectome (GT) and fit error. Finally, we examined the effects of the reduced redundancy by testing the robustness to perturbations in the input (Fig.3-A). We performed the fit 10 times, using optimal parameters, and varying the Gaussian noise (SNR=30) added to the ground-truth density map; to compare the results, we computed the L1 distance and the coefficient of variation for both the estimated streamline weights (normalized distances from the mean) and connectomes (distances from GT).
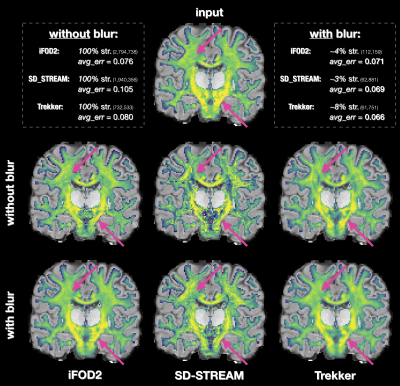
We also evaluated in vivo the effectiveness of COMMITblur on one HCP14 subject. We performed the fit using COMMIT without and with blurred streamlines and we compared the predicted intra-cellular compartment maps (IC) at whole-brain level (computing also the mean absolute difference from the input) and for two well-known bundles, Corpus Callosum and Pyramidal Tract.
Results and discussion
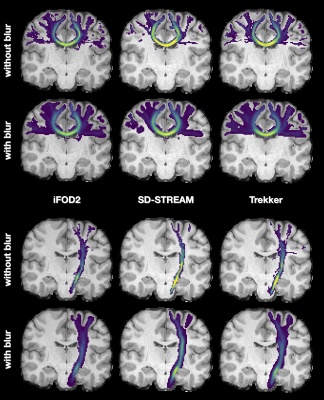
Fig.2 summarizes the results of the experiments performed on synthetic data; due to space constraints, we report only those corresponding to SD-STREAM. In panels B-C we see that increasing the clustering threshold leads to a substantial decrease in tractogram size (as expected) as well as the corresponding condition number. With thr=0.2mm we retain only 1% of streamlines and diminish the condition number by several orders of magnitude (≈1019$$$\rightarrow$$$≈103). Despite using so few streamlines, COMMITblur (optimal parameters, green square in D) is as accurate as COMMIT: L1_distance=21.5 from GT, RMSE=2.0x10-2, TP=27 and FP=65 (COMMIT: L1_distance=26.5, RMSE=1.4x10-2, TP=27, FP=178); the RMSE is slightly lower in COMMIT probably due to overfitting. Besides, Fig3-B shows that, thanks to the significant redundancy, COMMITblur produces estimates more robust estimated to small perturbations in the input.Fig.4 indicates that the blurred streamlines are effective also on in vivo data and it clearly shows that, despite using only ≈5% of the original streamlines, COMMITblur can predict the streamlines density (i.e. input intra-axonal signal map, top) more accurately and more homogeneously than without it. Additionally, looking at the results obtained from the three tractography algorithms, the predicted density maps appear closer between them when using COMMITblur, in particular for individual well-known bundles (Fig.5).
Future work will be needed to evaluate the impact of parameter choices on the estimated values and to assess the benefit of our approach in real clinical applications.
Conclusion
With this work, we wanted to raise awareness of the excessive (but always overlooked) redundancy present in typical tractograms and its consequences when using methods to filter them. To mitigate it, we developed an original representation called “blurred streamlines” which can effectively reduce redundancy and strengthen the robustness to perturbations in the input. All this is possible using only ≤5% of the original streamlines, thus decreasing storage requirements and computational complexity, while maintaining the same accuracy of state-of-the-art methods.Acknowledgements
References
1. Maier-Hein KH, Neher P, Houde JC, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8(1):1-13.
2. Daducci A, Dal Palú A, Descoteaux M, Thiran JP. Microstructure Informed Tractography: Pitfalls and Open Challenges. Front Neurosci. 2016;10:247.
3. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298-312.
4. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. 2015;119:338-351.
5. Daducci A, Dal Palù A, Lemkaddem A, Thiran J. A convex optimization framework for global tractography. In: 2013 IEEE 10thInternational Symposium on Biomedical Imaging. ; 2013:524-527.
6. Daducci A, Dal Palù A, Lemkaddem A, Thiran J. COMMIT: Convex Optimization Modeling for Microstructure Informed Tractography. IEEE Trans Med Imaging. 2015;34(1):246-257.
7. Schiavi S, Ocampo-Pineda M, Barakovic M, et al. A new method for accurate in vivo mapping of human brain connections using microstructural and anatomical information. Sci Adv. 2020;6(31):eaba8245.
8. Ocampo-Pineda M, Schiavi S, Rheault F, et al. Hierarchical microstructure informed tractography. Brain Connect. 2021;11(2):75-88.
9. Pestilli F, Yeatman J, Rokem A, Kay K, Wandell B. Evaluation and statistical inference for human connectomes. Nat Methods. Published online 2014:1058-1063.
10. Garyfallidis E, Côté MA, Rheault F, Descoteaux M. QuickBundlesX: sequential clustering of millions of streamlines in multiple levels of detail at record execution time. ISMRM. Published online 2016.
11. Tournier JD, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In: ISMRM. Vol 1670. ; 2010.
12. Tournier JD, Smith R, Raffelt D, et al. Mrtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137.
13. Aydogan DB, Shi Y. Parallel Transport Tractography. IEEE Trans Med Imaging. 2021;40(2):635-647. Doi:10.1109/TMI.2020.3034038
14. van Essen DC, Ugurbil K, Auerbach E, et al. The Human Connectome Project: A data acquisition perspective. Neuroimage. 2012;62(4):2222-2231. Doi:https://doi.org/10.1016/j.neuroimage.2012.02.018
Figures