5003
Feasibility of fast single-phase volumetric cardiac MR-thermometry1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom, 3School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 4Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Keywords: Myocardium, Thermometry
A novel fast simultaneous multi-slice black blood cardiac thermometry sequence is presented which allows multiple slices to be acquired in the same cardiac phase. A double inversion recovery pre-pulse is introduced to allow blood suppression and to optimise the pre-pulse strategy and therefore reduce acquisition time. Simultaneous multi-slice (SMS) imaging is integrated to further reduce the acquisition time of the multiple slices. Thermometry results obtained with this technique show an average temperature stability of 1.0±0.4°C in healthy subjects.Introduction
Catheter based ablation therapy has become a well-established treatment option for patients suffering from cardiac arrhythmias and is used to block abnormal electrical signals in the diseased tissue. MRI-guidance of such procedures represents a promising approach to improve procedural outcome, which currently has a high recurrence rate of up to 50% 1. MRI enables real-time temperature mapping in the heart and prediction of the extent of the ablation lesions 2,3. Current cardiac MR-thermometry methods cover the ablation region by acquiring multiple contiguous slices (usually Nslices=3-4) per heartbeat. These acquired slices, however, require pre-pulses such as fat suppression and saturation bands for in-flow blood saturation as well as reduced field of view imaging, in combination with a fast EPI readout. This leads to an acquisition time of around 300-400ms 4 per stack of slices, meaning that they are all acquired at different cardiac phases. Lesion assessment in the through-plane dimension is therefore severely limited 3, which is an important restriction of the method. This could lead to inadequate lesion formation which is known to cause a higher risk of developing recurrent arrhythmias following the procedure5. In this study, we sought to address these challenges by developing a fast single-phase volumetric cardiac MR-thermometry sequence. This was done by using simultaneous multi-slice (SMS) sequence combined with a more time efficient pre-pulse strategy for blood signal suppression.Methods
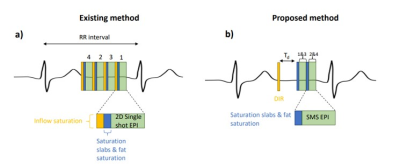
The proposed cardiac MR-thermometry sequence is shown in Figure 1. Four slices were acquired using an SMS single-shot EPI sequence with a multiband factor of 2. A double inversion recovery (DIR) pre-pulse was implemented for blood signal suppression instead of the typical in-flow saturation slabs that are commonly played before each slice. This DIR pre-pulse (thickness of the slice-selective re-inversion pulse=40mm, delay (Td) between DIR pulses and first SMS shot=225ms) therefore reduced the pre-pulse time taken before each slice whilst also delivering good dark blood signal suppression. Fat saturation pulses were used in both sequences prior to each SMS-EPI shot and saturation slabs were added to allow for a reduced field of view (FOV). This new prototype sequence was tested in five healthy subjects (4m, 1f) scanned on a 1.5T scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen Germany) under free breathing. The original thermometry sequence was also tested in two of the healthy subjects to be able to compare the magnitude images (shown in Figure 2). The common parameters for all scans were: TE=24ms, TR=63ms, Flip Angle=60o, Bandwidth=1420 Hz/px, FOV=250x250mm2, in-plane voxel size=3.1x3.1mm2, slice thickness=5mm, Nslices=4, multiband factor=2, number of dynamics=150, no slice gap. A multi-baseline approach using a look-up table of 40 dynamics, serving as a reference phase, was then used to reconstruct the motion corrected temperature maps offline. This was done for correction of respiratory induced phase variation together with non-rigid image registration, and temporal temperature filtering, as previously described 6. The stability of thermometry was assessed by calculating the standard deviation of the temperature in the myocardium over time. The stability in all 4 slices was combined and reported for each subject.Results
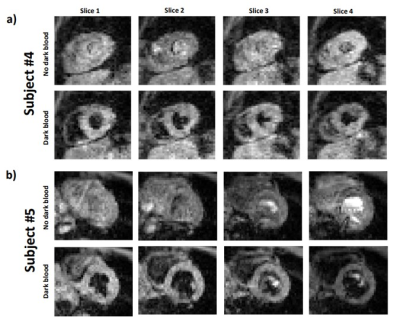
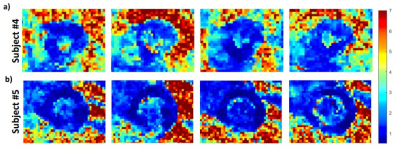
Successful blood signal suppression was achieved in all slices using the DIR pre-pulses, as can be seen in Figure 2 which shows typical images obtained for two subjects. Figure 3 displays the temperature map of the same two subjects over the 4 slices. The data from the subject shown in Figure 3b showed slightly better results with constant temperature stability values across the whole myocardium, averaging at under 1°C. The temperature map shown in Figure 3a represents the worst stability values obtained across the volunteers, averaging at around 1.4°C. The distribution of thermometry stability over the 4 slices of each volunteer is shown in the box plot in Figure 4. Over all the subjects, the thermometry stability was calculated to be 1.0±0.4°C.Discussion
The proposed approach demonstrates the feasibility of single-phase cardiac MR-thermometry with a precision of ~1°C. The blood signal suppression obtained in this study allows better visualisation of the morphologic features of the heart, which is important to improve the performance of the applied motion correction algorithm as well as the temperature stability 7. Only a limited number of subjects were included in this study and assessment of this technique on a larger cohort is warranted to confirm these findings. Since all the slices are acquired in the same cardiac phase, this technique has the potential to improve 3D assessment of ablation lesions, but further evaluation will be required during in-vivo ablation. Future work will also involve increasing in-plane spatial resolution and correcting for through-plane motion between heartbeats.Conclusion
The method proposed in this study allows multiple (Nslices=4) contiguous slices per heartbeat to be acquired in the same cardiac phase. This technique enables good blood signal suppression, and good thermometry stability of 1.0±0.4°C. The benefit of this technique for 3D lesion assessment will be the subject of future studies.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grants (EP/R010935/1), the Centre for Doctoral Training in Surgical and Interventional Engineering funded by King’s College London’s Centre for Doctoral Studies, the British Heart Foundation (BHF) grants (PG/19/11/34243 and PG/21/10539), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Kuck K. H., Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial Lancet (2010) 375:9708
2. Denis de Senneville B, Roujol S, Jais P, Moonen C, Herigault G and Quesson B. Feasibility of fast MR-thermometry during cardiac radiofrequency ablation NMR Biomed (2012) 25:556-62
3. Toupin S, Bour P, Lepetit-Coiffe M, Ozenne V, Denis de Senneville B, Schneider R et al. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J Cardiovas Magn Reson (2017) 19:14
4. Ozenne V, Toupin S, Bour P, D. de Senneville B, Lepetit-Coiffe M, Boissenin M, et al. Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation Magn Reson Med (2017) 77:673-683 DOI: 10.1002/mrm.26158.
5. Sacher F, Tedrow U. B., Field E, Raymond J. M., Koplan B. A., Epstein L. M., et al. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol (2008) 1:3
6. Roujol S, Ries M, Quesson B, Moonen C, Denis de Senneville B. Real-time MR-thermometry and dosimetry for interventional guidance on abdominal organs. Magn Reson Med. (2010) 63:1080–7.
7. Hey S, Cernicanu A, de Senneville B. D., Roujol S, Ries M, Jais P, Moonen C.T.W and Quesson B. Towards optimized MR thermometry of the human heart at 3T. NMR Biomed (2011) 25:1
Figures