5000
Susceptibility artifacts during MR-guided laser interstitial thermal therapy: characterization and temperature-based control solutions1Politecnico di Milano, Milan, Italy, 2IHU Strasbourg, Institute of image-guided surgery, Strasbourg, France, 3Siemens Healthcare SAS, Saint Denis, France, 4University of Utah, Salt Lake City, UT, United States
Synopsis
Keywords: Interventional Devices, MR-Guided Interventions
The use of Magnetic Resonance Thermometry (MRT) has been proposed to intraoperatively guide laser interstitial thermal therapy (LITT) thanks to its ability to provide multidimensional temperature measurements. PRF-based MRT thermometry holds significant benefits, but it is still limited by susceptibility artifacts that significantly affect the accuracy of measured temperature maps. These artifacts lead to a negative temperature distribution with a double-lobe shape around the laser applicator. In this work, artifacts appearing in MRT images during LITT and linked to magnetic field distortion related to susceptibility variations are characterized in ex-vivo livers. We further propose an approach to avoid their appearance.INTRODUCTION
Magnetic Resonance Thermometry (MRT) has been proposed to intraoperatively guide laser interstitial thermal therapy (LITT) thanks to its ability to provide multidimensional temperature measurements (1,2). During MR-guided LITT, the system software utilizes the measured temperature maps to estimate the thermal damage giving an estimation of the damage boundaries in real-time (3). Despite its potential, MRT is not yet established as the standard temperature monitoring method during LITT. PRF-based thermometry holds significant benefits (4), but some sources of errors, including susceptibility artifacts, affect the accuracy of temperature maps (5) due to the artifacts appearing with a double-lobe shape of temperature decrease (6, 7, 8) that we were indeed able to reproduce during LITT in the liver of a living pig (data not shown). In this work, artifacts appearing in MRT images during LITT and linked to magnetic field distortion related to susceptibility variations are characterized in non-homogeneous ex vivo livers. We then propose an alternative method to avoid their appearance.METHODS
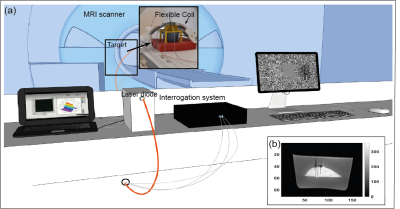
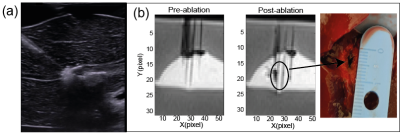
LITT was performed using a laser diode (975 nm, LuOcean Mini 4, Lumics, Berlin, Germany) delivering radiation to an MR-compatible fiber applicator (365 µm diameter, THORLABS, Dachau, Germany). MRI was performed with a 1.5T clinical MRI scanner (Siemens Magnetom Aera, Erlangen, Germany), spine coil and 4-channel flex surface coil (Figure 1), and temperature images were obtained with an MRT 3D segmented EPI prototype sequence. The data analysis was performed on Matlab R2020a after drift correction with zero-order baseline correction selecting a reference area in the target far from the ablation region and subtracting the effect of the non-temperature-related field drift (6). Series of ultrasound (US) images were acquired using a curvilinear transducer (Acuson S3000 + 6C1 transducer, Siemens Healthineers) to monitor tissue effects during LITT.Tissue mimicking phantoms
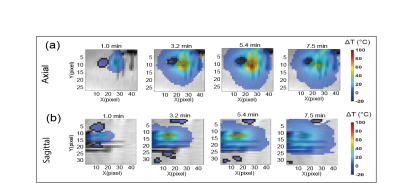
The artifacts were characterized in ex vivo porcine liver for 0.5W and 2W power. Data were acquired with the MRT sequence in the axial and sagittal orientation. Slices were placed parallel to the laser applicator and sensing needles. The following parameters were used: field of view 300 mm x 300 mm, in-plane resolution 1.4 mm x 2.8 mm, reconstructed resolution 1.4 mm x 1.4 mm, slice thickness 3 mm, 10 slices, TE/TR=13 ms/24 ms, flip angle 10°, EPI factor 7, 5 baseline averages to reach equilibrium magnetization, temporal resolution 3.62 s, 135 to 300 measurements leading to a total acquisition time of ~8 to 20 minutes. Areas of temperature errors were defined as zones of temperature decrease (<-2°C).
Temperature-based control LITT
Laser power was controlled via a custom-made program based on temperature values measured by MR-compatible sensing needles embedding Fiber Bragg Grating (FBG) array sensors. The laser power increased linearly and was then either maintained stable for a specific time duration, or until a maximum MRTI temperature change of 50°C was reached. MRTI data were acquired in the sagittal orientation to have slices parallel to the laser applicator and temperature sensing needles.
RESULTS
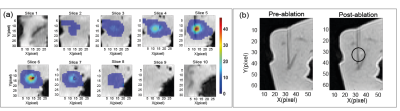
Artifacts in porcine liverAt 0.5W LITT heating the liver temperature maps do not show any artifacts. However, at a higher power of 2W, artifacts appear (Figure 2). As found in (6), gas bubbles were found to be formed in the tissue by reproducing the experiment with an ultrasound imaging protocol (Figure 3). In the axial plane, the area increases in the external slices during the ablation, whereas in the zone closest to the laser tip (Slice 6-8) it drops. In the sagittal case, a double-lobe of negative temperature shift is visible in the first steps of the ablation, then one region is more affected by susceptibility error. The artifact shifts to the right and starts decreasing after 3 minutes of ablation as in the axial case, probably because of the motion of the bubble. A spatial symmetry is less evident in this case.
Temperature-based control LITT
The temperature-controlled approach guaranteed the absence of artifacts during the liver interstitial ablation (Figure 4). The calculated temperature increase corresponds to absolute temperature in the range of 53-66°C for the tissue considering the starting temperature of 18°C in the ex vivo liver which causes thermal damage after a few minutes of treatment.The thermal damage induced after the treatment with a temperature-controlled strategy is visible in the MR images with a VIBE sequence.
DISCUSSION & CONCLUSION
This analysis confirmed the appearance of susceptibility artifacts during MR-guided LITT. Our investigation in tissue-mimicking phantoms linked the artifact appearance with gas bubble formation and with unwanted treatment effects which produce magnetic susceptibility changes previously reported in (6). Artifacts may be avoided when a low power of 0.5W is set, but such low power does not lead to tissue damage, which was confirmed with MRI images acquired after ablation. An alternative strategy may consist of temperature-controlled LITT to completely avoid any appearance of susceptibility artifacts. It allowed avoidance of bubble formation and appreciable but small tissue damage in the post-ablation MRI images. This is the first time that temperature-controlled LITT based on accurate FBGs values is performed inside an MRI scanner. Further investigations are needed to confirm these promising features.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant agreement No. 759159). The authors thank Dr. Sunil Patil (Siemens Medical Solutions, USA Inc, USA) and Dr. John Roberts (University of Utah, USA) for the development and optimization of the 3D EPI prototype.References
1. Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors – the NeuroBlate System. Expert Rev. Med. Devices 2014;11:109–119 doi: 10.1586/17434440.2014.882225.
2. De Landro M, Ianniello J, Yon M, et al. Fiber bragg grating sensors for performance evaluation of fast magnetic resonance thermometry on synthetic phantom. Sensors (Switzerland) 2020 doi: 10.3390/s20226468.
3. Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008;63:ONS21-8; discussion ONS28-9 doi: 10.1227/01.neu.0000335007.07381.df.
4. De Landro M, Korganbayev S, Ambarki K, et al. Magnetic resonance-based measurement system: comparison of 2D and 3D echo-planar imaging sequences for thermometry application. In: 2021 IEEE International Instrumentation and Measurement Technology Conference (I2MTC). ; 2021. pp. 1–6. doi: 10.1109/I2MTC50364.2021.9460088.
5. Winter L, Oberacker E, Paul K, et al. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016 doi: 10.3109/02656736.2015.1108462.
6. Viallon, M. et al. (2010) ‘Observation and correction of transient cavitation-induced PRFS thermometry artifacts during radiofrequency ablation, using simultaneous Ultrasound/MR imaging’, Medical Physics, 37(4), pp. 1491–1506. doi: 10.1118/1.3309439.
7. M. D. Landro et al., "Characterization of Susceptibility Artifacts in MR-thermometry PRFS-based during Laser Interstitial Thermal Therapy," 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), 2022, pp. 1-5, doi: 10.1109/MeMeA54994.2022.9856421.
8. M. De Landro et al., "Analysis of cavitation artifacts in Magnetic Resonance Imaging Thermometry during laser ablation monitoring," 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 2022, pp. 5008-5011, doi: 10.1109/EMBC48229.2022.9871675.
Figures