4999
PRF thermometry at 0.55T using multi-contrast segmented EPI approach1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Keywords: Thermometry, Multi-Contrast, Prostate, Brain
MR thermometry based on the proton resonance frequency (PRF) shift is a widely used tool to monitor changes in tissue temperature in response to MR-guided thermal interventions. However, MR temperature mapping at low field (< 1T) is challenging because of reduced signal to noise ratio (SNR) and intrinsically low temperature-sensitivity of the PRF shift. In this work, we present a multi-contrast segmented EPI sequence for PRF thermometry at low field. The proposed sequence provides high sampling efficiency and improves temperature quantification via echo combination. The performance of the sequence was tested in healthy volunteer measurements in the absence of heating.
Introduction
Low field MRI holds promise for interventional MRI due to reduced device and implant heating, RF power deposition, and susceptibility artifacts1. While MR thermometry is widely used in interventional MRI to monitor changes in tissue temperature in response to thermal therapy2,3, the precision of proton resonance frequency (PRF) thermometry suffers at low field because of reduced signal to noise ratio (SNR) and low temperature-sensitivity of MR phase4. In this abstract, we propose that multi-contrast segmented EPI is well suited for PRF thermometry at low field and demonstrate its performance in the brain and prostate at 0.55T.Methods
PRF thermometry is challenging at 0.55T because both SNR and temperature-induced phase shifts (temperature sensitivity ~0.01 ppm/°C) are inversely proportional to B0. However, one can compensate for the reduced temperature sensitivity by using longer TEs, which takes advantage of the longer T2* at reduced B0. Longer TRs, as necessitated by longer TEs, allow more T1 recovery and thus boost SNR, but result in poorer temporal resolution and less efficient data sampling when using conventional GRE sequences.A previous study exploited the longer TRs to combine segmented EPI with reduced bandwidth (BW) readouts to improve temporal resolution and thermometry precision at 0.55T4. As an alternative to reducing BW, the longer TRs can be filled in with multiple echo-trains to acquire images at multiple TEs. This helps achieve high sampling efficiency and improves thermometry precision via echo combination. Further, it provides relative insensitivity to variation in T2*, and lower spatial distortion as compared to EPI acquisitions with reduced BW or even longer echo-trains.
Data acquisition:
We implemented a multi-contrast segmented EPI prototype sequence on a 0.55T scanner (MAGNETOM Free.Max, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China). The sequence was tested and evaluated in a healthy volunteer in the absence of heating according to institutional volunteer scanning policies. Image series consisting of 50 repetitions were acquired in the brain and prostate using the protocols summarized in Figure 2.
Processing and Analysis:
Prior to phase difference calculation, images with significant subject motion were rejected. Baseline phase was removed using a principal component analysis based approach5 from individual echoes. Average phase over an SNR-based mask was removed to eliminate global phase drift. Resultant phase difference images were scaled by -1/(γB0 TE×0.01ppm/°C) to estimate temperature difference maps (∆T) relative to the first acquisition. ∆T maps from individual echoes were combined using an optimal weighted average approach6.
Since a temperature change of 0 is expected in absence of external heating, the temporal standard deviation (σ∆T) of the calculated ∆T series was used to estimate the precision of the PRF thermometry.
To enable the comparison between the single- and multi-contrast approaches, the following relationship between single-contrast σ∆T and TE with otherwise fixed imaging parameters was used:
$$\sigma_{\Delta T} = K e^{TE/T_2^*}/TE \tag{Equation 1}$$T2* and K were fitted using multi-contrast magnitude data and the estimated σ∆T maps respectively on a voxel-by-voxel basis. Equation 1 was used to simulate single-contrast σ∆T maps, referred to as σsim (TE=T2*), with sampling at optimal TE, i.e. TE = median T2* over organ of interest.
Results
All protocols with optimal echo combination resulted in a temperature precision ≤ 1°C (2D brain, 3D brain and 3D prostate: σ∆T = 0.8 °C, 0.5 °C and 1.0 °C respectively, Figures 3-5). Echo combination resulted in better ∆T precision as compared with individual echoes in all cases.Median T2* values for the brain and prostate were 85 ms and 99 ms, respectively. Average σsim (TE=T2*) values were 1.0 °C, 0.6 °C and 1.2 °C respectively for 2D brain, 3D brain and 3D prostate protocols, suggesting the superiority of multi-contrast protocols over equivalent single-echo protocols with TE = median T2*.
Discussion
Our results demonstrate that PRF thermometry based on multi-contrast segmented EPI can be achieved in the brain and prostate at 0.55T. The estimated brain T2* (85 ms) is consistent with existing literature1. We also report T2* value of the prostate at 0.55T (99 ms).The multi-contrast approach has key advantages over the single-contrast alternative. First, sampling at multiple TEs is beneficial when the region of interest includes voxels with a range of T2* values. For example, it is impossible to find a TE which simultaneously maximizes ∆T precision in the brain as well as extra-brain tissue. Sampling the magnetization at a range of TEs makes it possible to achieve high ∆T precision in multiple tissue types (Figures 3-5).
Second, while longer echo trains and reduced BW improve sampling efficiency and ∆T precision, these approaches increase spatial distortion. Our proposed multi-contrast approach similarly improves ∆T precision but does not sacrifice spatial fidelity. For instance, to achieve the same ∆T precision as the muti-contrast 2D brain protocol (Figure 2) on the same scanner, a single contrast protocol with the same TR/echo train length and TE=median brain T2* would require ~40% lower BW, resulting in ~55% increase in B0 distortion in phase-encode direction.
Third, multi-contrast imaging enables phase unwrapping along the TE dimension and will reduce temperature errors due to sudden resonance frequency changes between repetitions.
Future work will focus on validation and assessment of this approach for PRF thermometry of moving organs, such as liver.
Acknowledgements
No acknowledgement found.References
1) Campbell-Washburn, Adrienne E., et al. "Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI." Radiology 293.2 (2019): 384-393.
2) Rieke, Viola, and Kim Butts Pauly. "MR thermometry." Journal of Magnetic Resonance Imaging 27.2 (2008): 376-390.
3) Blackwell, James, et al. "Proton Resonance Frequency Shift Thermometry: A Review of Modern Clinical Practices." Journal of Magnetic Resonance Imaging (2020). doi: jmri.27446
4) Majeed, Waqas, et al. “Feasibility of Magnetic Resonance Thermometry at 0.55T”. Proc Intl Soc Magn Reson Med 2021.
5) Majeed, Waqas, et al. “A Principal Component Analysis based Multi-baseline Phase Correction Method for PRF Thermometry.” Proc Intl Soc Magn Reson Med 2019.
6) Odéen, Henrik, et al. "Improved MR thermometry for laser interstitial thermotherapy." Lasers in surgery and medicine 51.3 (2019): 286-300.
Figures
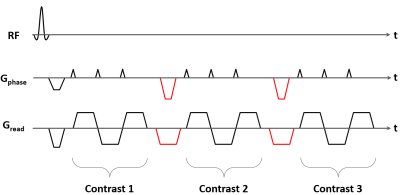
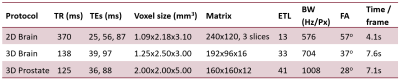
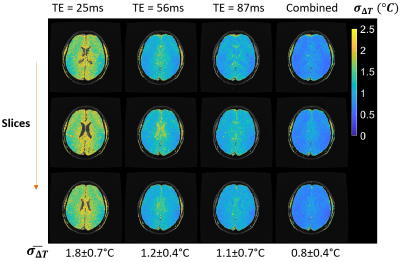
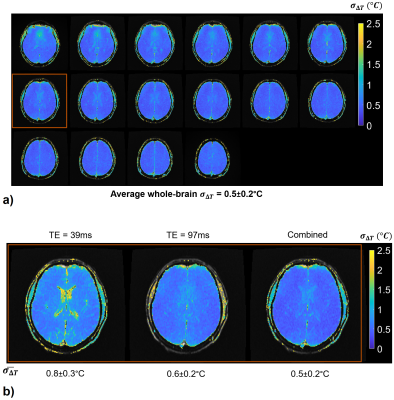
Figure 4:
a) σ∆T of echo-combined ∆T with the 3D brain protocol. σ∆T is less than 0.6°C for 80% of the brain voxels.
b) σ∆T of individual and echo-combined ∆T from the representative slice highlighted in 4 a). $$$\bar{\sigma_{\Delta T}}$$$ values represent average whole-brain σ∆T. Echo combination results in improved ∆T precision, as compared with individual echoes. Improved thermometry precision in extra-brain tissue at short TEs and ventricles at long TEs is preserved in the echo-combined ∆T.
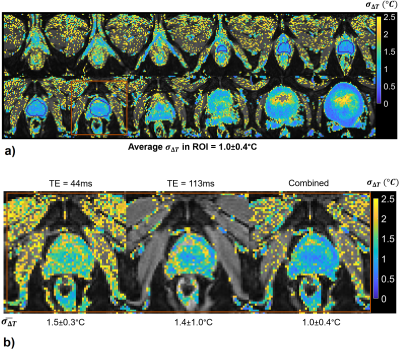
Figure 5:
a) σ∆T of echo-combined ∆T with 3D prostate protocol. σ∆T is less than 1°C in 60% of the voxels within the indicated ROI. Slices are cropped to show details.
b) σ∆T of individual and echo-combined ∆T from a representative slice. $$$\bar{\sigma_{\Delta T}}$$$ values represent average σ∆T over the ROI show in Figure 5a). Improved thermometry precision in the tissue outside the prostate at short TEs and the prostate at long TEs is preserved in the echo-combined ∆T.