4998
Low field cardiac MR thermometry: feasibility at 0.55T1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom
Synopsis
Keywords: Myocardium, Thermometry
Low field strength MRI scanners are appealing for MR guided interventions because of their lower associated costs and increased compatibility with interventional devices. Cardiac PRFS thermometry shows promise for real-time guidance of ablation therapy of cardiac arrhythmias. In this study, initial characterization of cardiac PRFS thermometry at 0.55T was performed in 5 healthy volunteers by measuring the stability over time. The stability of thermometry was 1.6±0.8 °C in the myocardium, confirming feasibility at this field strength.
Introduction
Low field MRI (<1 T) is of interest for MR-guided interventions compared to higher field strengths as more devices can safely be introduced whilst reducing the artifact level they have on images, and the associated costs are lower1. One such intervention is catheter ablation of cardiac arrhythmias. Cardiac MR thermometry with the proton resonance frequency shift (PRFS) method is promising for guidance of this procedure as it enables monitoring of myocardial tissue temperature in real-time, which can be used to characterize ablation lesions as they are forming2,3,4. On the other hand, SNR is intrinsically lower at lower field strength and the precision of PRFS thermometry has an inverse linear dependency on field strength as well5. This may be (partially) compensated by the longer echo times available due to increased T2* values (e.g. myocardium: 47±4 at 0.55T vs. 30-37 at 1.5T1). Here, we present the initial feasibility study of cardiac PRFS thermometry at 0.55T.Methods
To assess the feasibility of cardiac MR thermometry at low field, a modern 0.55T scanner (MAGNETOM Free.Max, Siemens Healthcare, Erlangen, Germany) was used to scan five healthy subjects (2f, 3m) using the PRFS method5. Images of the left-ventricular myocardium were acquired in short axis orientation in the diastolic cardiac phase using a prototype ECG-triggered (Invivo, Orlando, FL) single-shot EPI sequence. The scan parameters were: TE = 40 ms, TR = 76 ms, BW = 1086 Hz/px, FOV = 350x350 mm2, in-plane voxel size = 2.7x2.7 mm2, slice thickness = 6 mm, slices = 3, GRAPPA factor = 2, flip angle = 75°, echo train length = 66 ms, number of dynamics = 300. EPI distortion correction was applied.Temperature maps were generated offline using a post-processing pipeline6 that consisted of a multi-baseline correction of respiratory induced phase variations (look-up-table length = 30 dynamics), non-rigid image registration for motion correction, and temporal filtering to reduce noise. The stability of thermometry was calculated for each voxel inside the myocardium as the standard deviation over time of thermometry values and was reported per subject and over all subjects.
Results
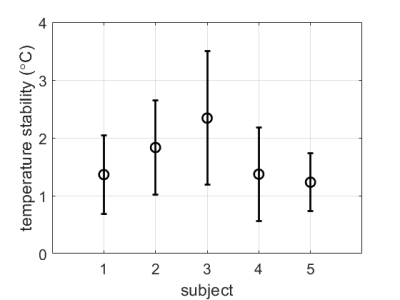
Cardiac MR thermometry images were successfully acquired in all subjects. Example images from subject #5 are shown in Figure 1. Some remaining distortions can be seen near the interface of the myocardium with the lung and liver. Temperature stability was comparable over the entire myocardium and in all slices.Figure 2 shows the stability of thermometry per subject, reported as mean±SD over all myocardial voxels in the 3 acquired slices. Over all subjects, the thermometry stability was 1.6±0.8 °C.
Discussion
Cardiac PRFS temperature stability at 0.55T is similar to previously reported stability of 1.5±0.4 °C at 1.5T3 and is promising for guiding cardiac ablations. Image distortion, slice coverage, and spatial resolution could be improved and will be the topic of further studies. The use of reduced field of view imaging and blood signal suppression techniques, as currently used at 1.5T, may be combined with the current approach to further improve MR-thermometry, and will also be explored in future work.Conclusion
Cardiac MR thermometry in the left ventricle is feasible at 0.55T with a stability of 1.6±0.8 °C, which is promising for the guidance of cardiac ablations.Acknowledgements
This work was supported by the Innovate UK grant (68539), the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the British Heart foundation (BHF) grants (PG/19/11/34243 and PG/21/10539), the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Campbell-Washburn, AE, et al. (2019). Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology 293(2): 384-393.
2. Denis de Senneville B, Roujol S, Jaïs P, Moonen CT, Herigault G, Quesson B. Feasibility of fast MR-thermometry during cardiac radiofrequency ablation. NMR Biomed. 2012 Apr;25(4):556-62.
3. Toupin S, Bour P, Lepetit-Coiffé M, Ozenne V, Denis de Senneville B, Scneider R. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J Cardiovasc Magn Reson. (2017) 19:14.
4. Mukherjee RK, Roujol S, Chubb H, Harrison J, Williams S, Whitaker J, et al. Epicardial electroanatomical mapping, radiofrequency ablation, and lesion imaging in the porcine left ventricle under real-time magnetic resonance imaging guidance—an in vivo feasibility study. EP Eur. (2018) 20:f254–62.
5. Rieke V, Pauly KB. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390.
6. Roujol S, Ries M, Quesson B, Moonen C, Denis de Senneville B. Real-time MR-thermometry and dosimetry for interventional guidance on abdominal organs. Magn Reson Med. (2010) 63:1080–7.