4993
Validation of an MR Thermometry Motion Compensation Algorithm for Periodic Motion During MR guided High Intensity Focused Ultrasound (MRgHIFU)1Posluns Centre for Image Guided Innovation & Therapeutic Interventions, Hospital for Sick Children, Toronto, ON, Canada, 2Department of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 3Department of Medical Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Motion Correction, Thermometry
Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) can noninvasively administer controlled hyperthermia as an adjuvant cancer therapy. For clinical translation, one of the main challenges is the sensitivity of MR thermometry to motion artifacts. This work aims to validate a real-time hybrid principal component analysis and projection onto dipole fields (PCA-PDF) motion compensation algorithm on a clinical MRgHIFU system during reproducible motion profiles. The real-time PCA-PDF algorithm maintained a temperature standard deviation of < 1°C in a phantom while the periodic motion was induced on a phantom using an MR-compatible robot.Introduction
Standard-of-care cancer therapies, such as radiation and chemotherapy, can have improved efficacy when combined with mild hyperthermia in more resistant tumour types1-3. One localized, non-invasive method of administering mild hyperthermia is magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU). MRI is used for treatment planning and monitors the treatment with magnetic resonance thermometry, which maps temperature changes overlaid on the anatomical images. However, one of the main challenges of MR thermometry is that it is highly sensitive to motion artifacts, which can skew temperature measurements4. Real-time motion compensation algorithms can reduce temperature uncertainty during treatment by removing any artifacts that appear in the thermometry when motion occurs. The motion artifact removal algorithm used in this work is a hybrid of principal component analysis and projection onto dipole fields (PCA-PDF)5. The objective of this study was to validate the capabilities of the PCA-PDF motion compensation algorithm under a controlled periodic motion profile for hyperthermia on a clinical MRgHIFU system.Methods
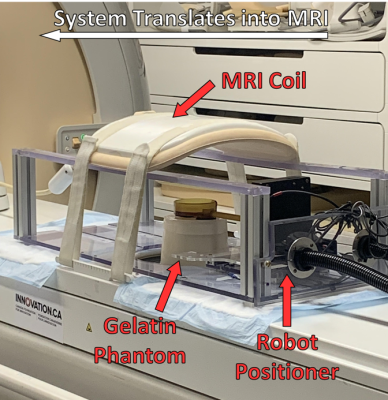
The motion compensation validation was done on a clinical MRgHIFU system consisting of a 3T Achieva MRI (Philips Healthcare, Best, NL) and Sonalleve V1 HIFU (Profound Medical, Toronto, Canada) system. For the MR thermometry, an echo-planar fast-field echo imaging (EPI-FFE) sequence was used. A custom closed-loop hyperthermia software called Proteus6 was used for implementing the PCA-PDF algorithm. An MR-compatible robot was constructed to allow movement across the right-left (RL) and superior-inferior (SI) directions while not impeding ultrasound propagation. To recreate periodic motion, a sinusoidal movement with a period of 4s and an amplitude of 10mm was used, which was found to be the general movement of the diaphragm in healthy patients7. This robot was designed to induce motion on custom phantoms fabricated using 10% gelatin and 2% silica (Figure 1). This formulation was ideal to simulate a tissue-like stiffness which shows a speed of sound to 1540m/s, the average speed of sound through human tissue and bulk attenuation of 1 dB/cm/MHz8. In initial tests, no sonications were performed, to remove all temperature-dependent phase changes. In doing so, we can conclude the thermal signal present during the motion to be solely due to motion artifacts. During the atlas acquisition of 20 images, the robot moved at the same periodic specifications as during the MR thermometry sequence. No motion was induced for the first 2 minutes once the thermometry started, to act as a baseline. For the following 2 minutes, the periodic motion was induced on the phantom with the robot within the MRgHIFU system. After, an additional 2 minutes of the thermometry sequence was acquired without movement. This was performed 5 times without motion compensation where the PCA-PDF algorithm would be run retrospectively and 5 times with the real-time PCA-PDF algorithm. These protocols were repeated with hyperthermia administered by the MRgHIFU system. To analyze the thermometry data, the standard deviations of temperature measurements were calculated for each voxel over the thermometry images when motion was induced on the phantom. A paired t-test was run to compare the average temperature standard deviation between the original subtraction thermometry and both the retrospective and real-time PCA-PDF.Results
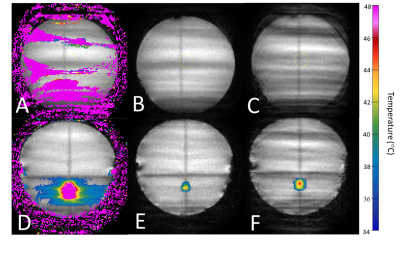
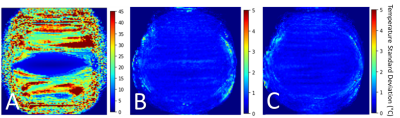
The spatial average temporal standard deviation in the thermometry data without motion compensation was 20.9°C ± 1.4°C. After the retrospective application of the PCA-PDF algorithm to these data sets, a substantial amount of motion artifacts were removed (Figure 2), similar to the trials where the PCA-PDF algorithm was run in real-time. The average temperature standard deviation with the retrospective PCA-PDF was reduced to 0.5°C ± 0.03°C. This is comparable to the real-time PCA-PDF test, which had an average temperature standard deviation of 0.4°C ± 0.05°C (Figure 3). The PCA-PDF algorithm improved MR thermometry by reducing temperature standard deviation with statistical significance (p<0.001).Discussion
We can create reproducible periodic motion by using a robot positioner for the phantom. Preliminary results demonstrated that the PCA-PDF algorithm compensated for periodic motion, significantly reducing motion artifacts. When hyperthermia was administered, the PCA-PDF algorithm was able to distinguish temperature-dependent phase changes from motion-induced phase changes. Even when substantial motion artifacts were present in the magnitude MRIs, the PCA-PDF algorithm was able to compensate successfully (Figure 2C). There was a decrease in temperature standard deviation from 20.9°C with the original subtraction thermometry method to <1°C with the retrospective and real-time PCA-PDF methods. These results verified that the PCA-PDF algorithm is comparable in both retrospective and real-time modes, confirming that we can rely on the results during treatment.Conclusion
The results demonstrate that the PCA-PDF algorithm's real-time capabilities were comparable to retrospective analysis, where the algorithm reduced temperature uncertainty to <1°C. Results also verified that the algorithm successfully distinguished heating from motion artifacts. Future work includes expanding the types of motion profiles to recreate more sporadic or arbitrary movements and further validation of the PCA-PDF algorithm.Acknowledgements
We thank Samuel Pichardo for providing access to the Proteus software and technical assistance. Funding is provided by the Natural Sciences and Engineering Research Council (NSERC).References
1. Datta NR, Puric E, Klingbiel D, Gomez S, Bodis S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073-1087. doi:10.1016/j.ijrobp.2015.12.361
2. Datta NR, Rogers S, Klingbiel D, Gómez S, Puric E, Bodis S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: a systematic review with conventional and network meta-analyses. Int J Hyperth. 2016;32(7):809-821. doi:10.1080/02656736.2016.1195924
3. Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483-492. doi:10.1001/jamaoncol.2017.4996
4. Rieke V, Pauly KB. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390. doi:10.1002/jmri.21265
5. Tan J, Mougenot C, Pichardo S, Drake JM, Waspe AC. Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic resonance thermometry. Magn Reson Med. 2019;81(1):195-207. doi:10.1002/mrm.27368
6. Zaporzan B, Waspe AC, Looi T, Mougenot C, Partanen A, Pichardo S. MatMRI and MatHIFU: Software toolboxes for real-time monitoring and control of MR-guided HIFU. J Ther Ultrasound. 2013;1(1). doi:10.1186/2050-5736-1-7
7. Gorman RB, McKenzie DK, Pride NB, Tolman JF, Gandevia SC. Diaphragm length during tidal breathing in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(11):1461-1469. doi:10.1164/rccm.200111-087OC
8. Ryan LK, Foster FS. Tissue equivalent vessel phantoms for intravascular ultrasound. Ultrasound Med Biol. 1997;23(2):261-273. doi:10.1016/S0301-5629(96)00206-2
Figures