4992
Compensation for Sporadic Motion in MR Thermometry Though a Hybrid Augmented Multi-Baseline and Near-Referenceless Approach
Arthur Akbulatov1, Suzanne M Wong1,2, Craig A Macsemchuk1,2, Andrew Headrick1, James M Drake1,2, and Adam C Waspe1,3
1Posluns Centre for Image Guided Innovation & Therapeutic Intervention, The Hospital for Sick Children, Toronto, ON, Canada, 2Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 3Department of Medical Imaging, University of Toronto, Toronto, ON, Canada
1Posluns Centre for Image Guided Innovation & Therapeutic Intervention, The Hospital for Sick Children, Toronto, ON, Canada, 2Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 3Department of Medical Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Motion Correction, Motion Correction
Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatments are limited by motion artifacts introduced into MR thermometry calculations by large sporadic motions. A hybrid principal component analysis and projection onto dipole fields (PCA-PDF) motion compensation algorithm was expanded upon to include predicted motion using image augmentation techniques. When tested on MR images from a gelatin phantom being translated in the coronal plane, the improved PCA-PDF algorithm yielded a temperature standard deviation of 0.4 ± 0.1 °C, which is a 3.7 ± 0.1 °C reduction from an unmodified PCA-PDF approach, indicating that thermometry artifacts induced by motion were significantly reduced.Introduction
High-intensity focused ultrasound (HIFU) is a non-invasive technique used for tissue hyperthermia. In combination with magnetic resonance (MR) thermometry, magnetic resonance-guided HIFU (MRgHIFU) is capable of treatment planning, targeting, and monitoring temperature changes within tissue in real time1. A significant clinical barrier for MRgHIFU is patient motion during treatment, which can impact thermometry measurements by introducing imposing thermometry artifacts2. Maintaining accurate thermometry measurements is essential to provide precise and accurate heating to regions of interest and avoid damaging tissue outside of the treatment area1. Compensation for periodic motion such as breathing and small aperiodic motion such as peristalsis has been previously demonstrated by Tan et al2 using a hybrid principal component analysis (PCA) and projection onto dipole fields (PDF) method, referred to as PCA-PDF. However, preliminary work performed using this implementation found that it compensated poorly for large aperiodic motions. Involuntary sporadic motions such as muscle twitching and sternutation can introduce large motion artifacts that severely impact the accuracy of MR thermometry, even when PCA-PDF is used. The objective of this work is to improve the PCA-PDF method to minimize thermometry artifacts caused by large unpredictable motions.Methods
The principal component analysis algorithm was expanded upon to incorporate predicted motion into the PCA atlas using image augmentation techniques. Prior to treatment, an atlas of MRI images is acquired to create the PCA reference. Each phase image acquired is augmented to create several new images that are added to the PCA atlas. These images are horizontally and vertically translated by a random number of pixels drawn from a Gaussian distribution centered at 0 with a specified pixel standard distribution. The new images serve to mimic motion that the subject could undergo during the treatment. After the atlas is expanded, the PCA-PDF method is carried out normally.To assess the efficacy of the algorithm, MRI images of a gelatin phantom undergoing lateral displacement were retrospectively processed in Proteus. Proteus is a vendor-agnostic software platform that interfaces with MRI and HIFU hardware, in which custom MR thermometry processing techniques were implemented3. MRI data was collected using an echo-planar fast-field echo imaging (EPI-FFE) sequence implemented on a 3T Achieva Tx MRI System (Philips Healthcare, Best, NL). The gelatin phantom imaged had a volume of 2L and was composed of 8% gelatin, 2% silica, and 0.1% formalin in distilled H2O. The gelatin phantom was translated by a robot positioner shown in figure 1 by approximately 2.25 cm – corresponding to 15 voxels when imaged with a voxel size of 1.5 mm – then moved back to its original position. Across all trials, 16 atlas images were acquired before motion. The number of augmented images created and the standard deviation of created image displacement were varied in each set of retrospective processing. As a measure of severity of artifacts, the standard deviation of temperature was calculated in each thermometry image where the phantom was in the displaced position and averaged to obtain a temperature standard deviation value for each trial.
Results
The temperature standard deviation resulting from a different number of atlas images and standard deviation of augmented image displacement is shown in figure 2. Figure 3 shows further trials with a displacement standard deviation of 10 pixels (column 2 of heatmap), which gave the lowest temperature standard deviation on average. The trials using 64-160 atlas images after augmentation showed a statistically significant reduction in temperature standard deviation compared to the atlas with 16 images. The 16-image atlas, produced with no augmentation, yielded a temperature standard deviation of 4.1 °C. The 128-image atlas yielded the lowest temperature standard deviation of 0.4 ± 0.1 °C, which is 3.7 ± 0.1 °C lower than the unmodified PCA-PDF implementation. The thermometry calculations resulting from this set of parameters are shown in figure 4, where an improvement in the severity of motion artifacts is evident.Discussion
For the gelatin phantom motion data collected, an atlas of 64-160 images resulted in a significantly lower temperature standard deviation (figure 3), indicating that artifacts resulting from aperiodic motion were minimized as a result of atlas augmentation. The qualitative effects of the motion compensation can be seen in the difference in temperature calculations between the two rows. The top row, in which the unmodified PCA-PDF method is used, has random, high temperature artifacts scattered throughout the entirety of the phantom. The augmented PCA-PDF method resulted in reduced temperature artifacts that would be easily distinguishable from induced focused ultrasound heating.Conclusion
The results obtained indicate that supplementing the PCA-PDF method with an augmented atlas of predicted motion images may be able to reduce artifacts created by sporadic motion in real-time MR thermometry. This would allow hyperthermia treatments using MRgHIFU to continue despite a large patient movement rather than being forced to stop and resume due to thermometry artifacts. This work also permits the development of more robust motion compensation algorithms for MR thermometry that can account for a wider range of bulk motions such as deformation, rotation, and more. Future work will involve optimizing this implementation to minimize computation time and further eliminate artifacts.Acknowledgements
We thank Samuel Pichardo for providing access to the Proteus software and technical assistance. Funding for this project was provided by the Natural Sciences and Engineering Research Council (NSERC), the Canadian Institutes of Health Research (CIHR), and the Canada Foundation for Innovation (CFI).References
1. F. A. Jolesz and K. Hynynen, MRI-guided focused ultrasound surgery. New York: Informa Healthcare, 2008.
2. J. Tan, C. Mougenot, S. Pichardo, J. M. Drake, and A. C. Waspe, “Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic resonance thermometry,” Magnetic resonance in medicine, vol. 81, no. 1, pp. 195–207, 2019, doi: 10.1002/mrm.27368.
3. S. Pichardo et al., “Proteus: A software platform for multisite development of MRI-guided focused ultrasound applications,” in Proc. ISTU, Nashville, TN, USA, 2018, pp. 137–139.
Figures
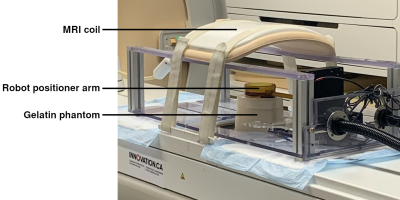
Figure 1: Experimental setup used to collect MRI data during motion. The gelatin phantom is positioned on the MRgHIFU table with the robot positioner overtop.
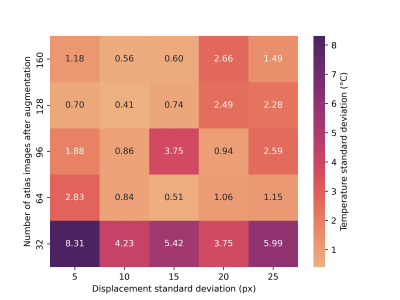
Figure 2: Heatmap of temperature standard deviation with varied displacement standard deviation and number of atlas images. Before augmentation, each trial collected 16 atlas images. Each square represents the average temperature standard deviation of five trials with the associated parameters.
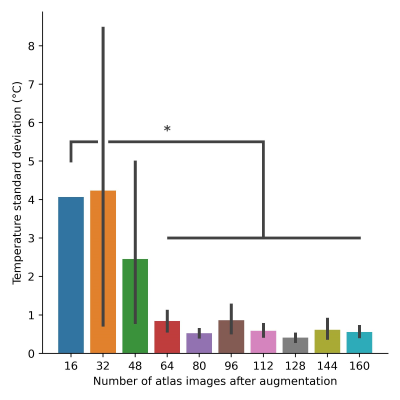
Figure 3: Temperature standard deviation calculated with varied number of PCA atlas images after augmentation. Displacement standard deviation is 10 pixels. *p<0.0001.
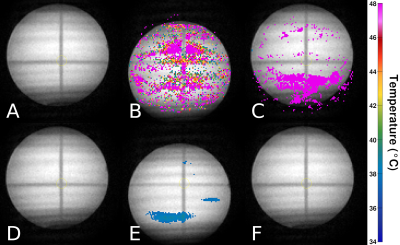
Figure 4: T1-weighted MRI magnitude images with thermometry data overlaid on top. No heating was induced. A-C: Temperature calculated using PCA atlas with 16 images (no augmentation). D-F: Temperature calculated using PCA atlas with 128 images and displacement standard deviation of 10 pixels. A, D: Before motion. B, E: After displacement away from original position. C, F: After displacement back to original position.
DOI: https://doi.org/10.58530/2023/4992