4979
Patient specific respiratory motion correction for cardiac MRI1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2United Imaging Healthcare, Shanghai, China, 3UIH America, Inc., Houston, TX, United States, 4Shanghai Clinical Research and Trial Center, Shanghai, China
Synopsis
Keywords: Heart, Motion Correction
Magnetic resonance imaging (MRI) is susceptible to respiratory motion-induced artifacts due to the relatively slow data acquisition. These artifacts remain an impediment to clinical application and are further amplified in cardiovascular MRI. Respiratory motion model is an effectively method to reduce motion artifacts. In this study, we proposed a novel patient-specific respiratory motion correction method with more reliable training data and capability to perform spoke-wise correction. Combined with compressed sensing, parallel imaging, image registration and model fitting, this method greatly reduces respiratory motion artifacts.Introduction
Cine cardiovascular MRI (CMR) is susceptible to respiratory motion-induced artifacts due to the relatively slow data acquisition. However, for a considerable number of patients, breath-holding, the preferred strategy to avoid respiratory motion, is not feasible. To allow free‐breathing image acquisition, respiratory motion model has been developed over the past 20 years1-3. A motion model can be defined as a process that takes certain surrogate data as input and produces a motion estimate as output4. Due to the respiratory variability between patients, in many proposed model techniques, a pre-scan procedure is required to form a patient-specific model. During pre-scan, real-time images of heart for motion estimation were acquired along with corresponding surrogate data, the estimated motion is then fitted to the surrogate data to establish the model2,3,5. Nevertheless, the real-time images suffer from motion artifacts itself due to relatively long acquisition time (more than 200ms), which reduces the reliability of training data. Moreover, although diaphragm navigator signal has been confirmed to be an effective surrogate data, limited by the sampling rate, all these methods cannot perform a spoke-wise correction. In this study, we proposed a novel patient-specific respiratory motion correction method with more reliable training data and capability to perform spoke-wise correction.Methods
Volunteers (24 $$$\pm$$$ 4 years, 3 males) data were collected with a free-breathing golden-angel radial sequence on a clinical 3T system (uMR890, United Imaging Healthcare, Shanghai, China).The pipeline of patient-specific motion correction technique is shown in Figure 1. A pre-scan procedure was performed at the beginning of the exam to acquire real-time images of the heart, based on which the end-diastole images were extracted along with the respiratory signal measured by respiratory bellows that was acquired at the time points corresponding to each end-diastolic image frame. To accommodate for hysteresis, the imaging data was classified as inhalation and exhalation. Total variation along temporal dimension could effectively enforce the sparsity for compressed sensing reconstruction of continuously acquired multi-frame images6-8. To make temporal total variation a feasible sparse transformation, the imaging data was further sorted based on the amplitude of bellows signal. Using compressed sensing and parallel imaging, we reconstructed each of the end-diastolic “snapshot” images. Retrospective 2D cross-correlation amongst the snapshot images was used to extract spatially resolved motion information based on rigid image registration. The translational motion of each snapshot image was then fitted to the corresponding respiratory signals, hence establishing the model, as illustrated in Figure 2. During the ‘live’ scan, the established model was applied to perform spoke-wise rigid motion correction for intra-plane motion and through-plane motion.
Although our approach could potentially be used to track through-plane motion in real time, in this study, we only evaluated the intra-plane motion to explore the feasibility of the proposed method. Data were acquired from sagittal view to capture the dominant Head-Foot and Anterior-Posterior respiratory motion. The acquisition of pre-scan data took 2 minutes. During the ‘live’ scan, all the k-space radial spokes were sorted according to their time stamps relative to the R-wave of the ECG, and spokes that belong to a selected cardiac phase are binned together. Motion model established during the pre-scan was then applied to individual k-space spokes for spoke-wise respiratory motion correction since the sampling rate of respiratory bellows is sufficiently high. This procedure was repeated for each cardiac phase until the average R-R interval is entirely covered, finally forming a respiratory corrected, segmented cine image that covers the entire cardiac cycle.
To analyze performance, the corrected data was retrospectively processed to regenerate a series of end-diastolic “snapshot” images to extract the ‘residual’ motion, hence compared with the ‘original’ motion. To provide a measure of displacement, RMS error (RMSE) is defined as
$$RMSE\;=\;\sqrt{\sum_{k=1}^{100}(d(k)-\bar{d})^{2}},$$
where$$$\;d(k)\;$$$represents a single magnitude measure of the motion at each frame,$$$\;\bar{d}\;$$$represents the value of$$$\;d(k)\;$$$averaged over all frames. An uncorrected, segmented cine was also formed with original data without motion correction for image quality comparison.
Results
Hysteresis could be clearly observed from the estimated translations, and third-order polynomial fitting well on the training data, as shown in Figure 3. In Figure 4, the original motion and residual motion after correction were shown together, and statistical analysis (Mean, SD, Range and RMSE) of the motion was recorded in the table. Residual motion was reduced compared to the original motion in almost every frame, and the bulk residual motion was only 53% of original motion. Figure 5 shows the performance of patient-specific model in reducing motion artifacts in segmented cine. After correction, the cine image became sharper, and more details can be resolved from the image. Due to the lack of ground truth and relatively obvious improvement, only visual assessment was performed on image quality.Discussion
Compressed sensing algorithm taking temporal total variation as the sparse transformation significantly improved the image quality with severe subsampling, as shown in Figure 2, which made it possible to get a snapshot with only 12 spokes (within 50ms). Relatively shorter acquisition time makes training data more reliable. The sampling rate of respiratory bellows is sufficiently high to perform spoke-wise correction. Compared with navigator-based methods, the bellows-based method proposed in this study has a higher theoretical performance limit.Acknowledgements
No acknowledgement found.References
1. Manke D, Rosch P, Nehrke K, Bornert P, Dossel O. Model evaluation and calibration for prospective respiratory motion correction in coronary MR angiography based on 3-D image registration. IEEE Transactions on Medical Imaging. 2002;21(9):1132-1141.
2. Burger I, Meintjes EM. Elliptical subject-specific model of respiratory motion for cardiac MRI. Magnetic Resonance in Medicine. 2013;70(3):722-731.
3. Bush MA, Ahmad R, Jin N, Liu Y, Simonetti OP. Patient specific prospective respiratory motion correction for efficient, free-breathing cardiovascular MRI. Magnetic Resonance in Medicine. 2019;81(6):3662-3674.
4. Mcclelland JR, Hawkes DJ, Schaeffter T, King AP. Respiratory motion models: A review. Medical Image Analysis. 2013;17(1):19-42.
5. Baumgartner CF, Kolbitsch C, Mcclelland JR, Rueckert D, King AP. Autoadaptive motion modelling for MR-based respiratory motion estimation. Medical Image Analysis. 2017;35:83-100.
6. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic Resonance in Medicine. 2007;58(6):1182-1195.
7. Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magnetic Resonance in Medicine. 2007;57(6):1086-1098.
8. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic Resonance in Medicine. 2014;72(3):707-717.
Figures
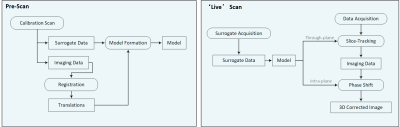
Figure 1: Block diagram illustrating the patient-specific motion correction. A pre-scan procedure was performed at the beginning to establish the respiratory motion model. During the ‘live’ scan, the established model was applied to perform spoke-wise rigid motion correction for intra-plane motion and inter-plane motion.
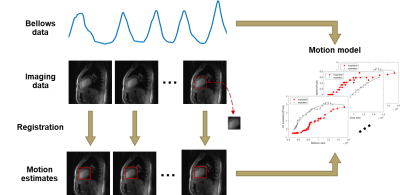
Figure 2: The formation of respiratory motion model. Bellows data was acquired at the same time as some imaging data that represents the heart at the same cardiac phase but different respiratory positions. Each frame of imaging data was acquired within 50ms, hence fast enough to freeze the respiratory and cardiac motion. A heart template was cropped from a random frame, and then registered to each of imaging data respectively based on 2D cross-correlation to estimate the motion. The motion model approximates the relationship between the bellows data and the motion.

Figure 3: Motionextraction from training data produces sets of $$$[n(k),dY_{i}(k),dX_{i}(k)]$$$ from sagittal view for k = 1:100, where $$$n(k)$$$ represents the bellows data and $$$dY_{i}(k),dX_{i}(k)$$$ the translation of heart. Each set is classified as inhalation and exhalation phase. Translations within each dimension and phase are then fitted to corresponding bellows data using third-order polynomial.

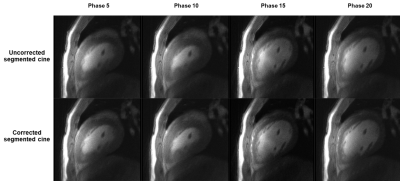
Figure 5: Comparison of uncorrected, segmented cine and corrected, segmented cine, 4 representative cardiac phases are shown here.