4977
Correcting motion-induced B0 shim failure at 3T CMR using a deep-learning-enabled 3D motion-resolved B0 shimming1Biomedical Image Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Krannert Cardiovascular Research Center, Indiana University School of Medicine, Indianapolis, IN, United States, 3Siemens Healthineers, Malvern, PA, United States
Synopsis
Keywords: Myocardium, Shims
B0 inhomogeneity is a long-lasting issue for CMR in high-field (3T and above) scanners. B0 shimming is a standard way to improve the B0 field. However, motion-induced field inhomogeneity is an unknown factor in routine practice and compromises cardiac B0 shimming quality. Here, we proposed a motion-resolved cardiac B0 shimming strategy by acquiring motion-resolved cardiac B0 field maps and adopting a modified U-net model for precise and automatic shim field derivation. We showed that dynamic shimming could improve field homogeneity through the respiratory cycle and provide a reliable B0 field for free-breathing CMR at 3T.Introduction
B0-field inhomogeneity has been a long-standing challenge at high-field (3T) CMR. State-of-the-art clinical scanners utilize active B0 shimming to improve B0 homogeneity. However, due to technical limitations, current cardiac field mapping sequences are commonly acquired with free-running acquisitions that do not mitigate cardiac or respiratory motion. Because respiratory motion introduces significant geometrical differences between the heart and lungs, it has been shown that the cardiac B0 field varies significantly through the respiratory cycle [1]. Besides the motion-induced B0 field variation, the current standard cardiac shimming protocol requires the operator to manually select a squared shim volume, which often falsely includes regions with significant B0 deviation (e.g., Liver and chest wall) through different motion states. The flawed shim field and shim volume can introduce imaging artifacts and compromise the reliability of CMR protocols. In this study, we combined a continuously acquired, motion-resolved B0 mapping sequence and a deep learning-based cardiac shimming model to reliably contour the cardiac shim volume without human interaction and enable motion-specific B0 shimming through free-breathing CMR acquisitions.Methods
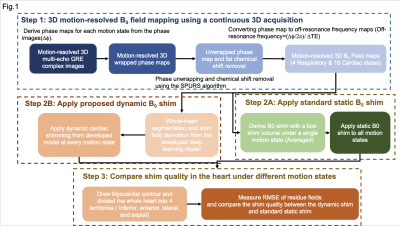
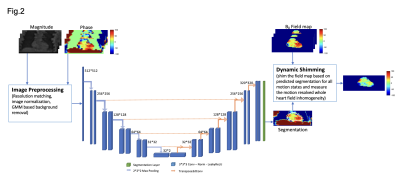
Healthy volunteers were studied with a clinical 3T scanner (Biograph mMR, Siemens). A Study flow chart is depicted in Fig. 1. Continuous 3D, motion-resolved multi-echo GRE (mGRE) was acquired (6 echoes, TE1/ΔTE =1.42/2.01ms). mGRE Images were reconstructed with 16 cardiac and four respiratory phases based on an LRT CMR framework [2]. Field maps of each motion state are derived following the image processing pipeline described in Fig. 1(Step1) [3]. To facilitate a motion-resolved cardiac shimming procedure, an automatic segmentation deep-learning model was developed to mitigate the motion-induced ROI difference for shim field derivation. The model is developed based on a two-channel UNet framework and is depicted in Fig. 2. The 3D deep learning model was trained with 5-fold cross-validation. The training set was composed of 3D field maps from 40 healthy subjects. Field maps covering the whole thoracic region were used. Notably, a Two channels model was adopted (utilizing the magnitude and phase of the field maps) to improve the segmentation quality on the low tissue contrast field mapping images. To test the proposed dynamic shimming pipeline, B0 shim fields were derived from the proposed dynamic shim and the standard static shim method using a 0-2nd order spherical harmonic shim field and applied to the motion-resolved B0 maps. (Fig1. step 2) The shim quality of each motion state was then measured with the normalized root mean square error (RMSE) of the residual field (Normalized RMSE=(RMSE after shim / RMSE before shim) × 100%). (Fig1. step 3) The static shim and dynamic shim were compared using a paired t-test.Results
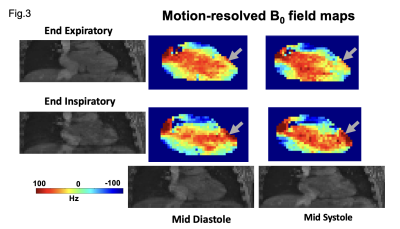
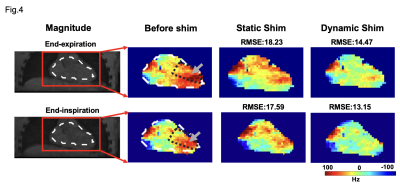
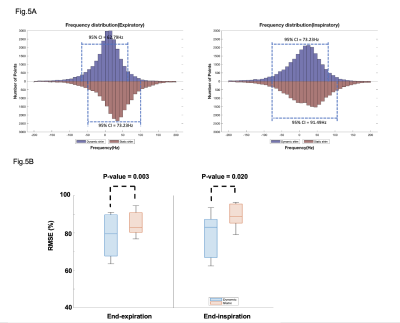
Representative coronal slices of the motion-resolved B0 field maps before shimming from a human subject are shown in Fig. 3. The field maps showed substantial B0 variation through the respiratory cycle(arrow) and remained relatively stable through the cardiac cycle. To investigate the effect of the proposed dynamic shimming pipeline, B0 shimming was conducted on the B0 maps under all respiratory states(end-inspiration to end-expiration) using the proposed dynamic shim method and the standard static shimming protocol. Representative shimming results are presented in Fig. 4. Significantly more homogeneous B0 field was achieved under dynamic shim in both respiratory states. Notably, the strong off-resonance field in the lateral region in the left ventricle (grey arrow) was successfully eliminated after applying dynamic shimming in both images. A quantitative analysis of the B0 fields post-shimming is presented in Fig. 5. The frequency histogram of the frequency distribution in the Lateral LV segment is shown in Fig. 5A. A tighter distribution and smaller 95% CI both in end-expiratory and end-inspiratory states were achieved post dynamic shimming. Furthermore, the normalized RMSE% of the post-shim fields are compared in Fig. 5B. Under both respiratory phases, the RMSE% is significantly lower under dynamic shimming compared to the static shim (both p-value<0.05). Remarkably, the dynamic shimming showed a stable improvement of the B0 field through the respiratory phases, which enables reliable B0 homogeneity for free-breathing acquisition CMR.Discussion
In the recent development of CMR, the free-breathing acquisition protocols have become an important approach to streamline image acquisition and accommodate patients with compromised breath-hold capability. High SNR efficient CMR sequence such as bSSFP is additionally sensitive to B0 inhomogeneity and require a homogeneous B0 field to avoid imaging artifacts. This study developed a motion-resolved cardiac B0 shimming framework by integrating a motion-resolved B0 mapping sequence and a deep-learning model for a free-running dynamic shimming pipeline through motion. Our results showed that the proposed framework could achieve a better and more robust cardiac B0 field under different motion states(especially in the lateral region of LV), which provides the essential condition for reliable free-breathing CMR at 3T.Conclusion
The developed dynamic cardiac shimming pipeline can eliminate the respiratory motion-induced field inhomogeneity in cardiac MRI. It can achieve a reliable B0 field across free-breathing acquisition and enable a reliable imaging condition for free-breathing CMR acquisitions at 3T.Acknowledgements
No acknowledgement found.References
[1]. Yuheng Huang, Xingmin Guan, Xinheng zhang, Xinqi Li, Ghazal Youseff, Xiaoming Bi, Fei Han, HsuLei Lee, Anthony Christodoulou, Debiao Li, Hui Han, Rohan Dharmakumar, Hsin-Jung Yang. The Effect of Respiratory and Cardiac Motion States on B0 shimming at 3T for Breathheld CMR of the Heart.
[2]. Xingmin Guan, Hsin-Jung Yang, Zhehao Hu, Nan Wang, Anthony Christodoulou, Behzad Sharif, Debiao Li, Rohan Dharmakumar, Free-breathing Fully Ungated 3D Cardiac T2* MR Mapping using a Low-Rank Tensor Framework, 2022 Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting(2022)
[3]. Dong J, Liu T, Chen F, Zhou D, Dimov A, Raj A, Cheng Q, Spincemaille P, Wang Y. Simultaneous phase unwrapping and removal of chemical shift (SPURS) using graph cuts: application in quantitative susceptibility mapping. IEEE Trans Med Imaging. 2015 Feb;34(2):531-40. doi: 10.1109/TMI.2014.2361764. Epub 2014 Oct 8. PMID: 25312917.
Figures