4976
Automatic catheter tracking during cardiac MRI guided cardiac catheterization using deep learning1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom
Synopsis
Keywords: Heart, MR-Guided Interventions
A deep neural network trained on images with artificially generated catheter signal was developed and enables accurate detection of catheter signal under real-time conditions. Further investigations, with in-line integration, are now warranted to determine the benefit of this technique for improved catheter navigation.Introduction
MRI-guidance is a promising substitute for traditional X-ray fluoroscopy in cardiac catheterization procedures1,2. Main advantages include improved soft tissue visualization, no ionization radiation exposure and improved hemodynamic data3. MRI-compatible gadolinium-filled balloon-wedge catheters are typically used in these procedures and are visually tracked using dynamic single-slice imaging from the corresponding hyper-intense signal of the catheter tip. During navigation, the catheter frequently goes out-of-plane, with a previously reported average balloon visibility of <70% of the scanning time4. The automatic segmentation of the catheter tip may enable enhanced visualization and thus facilitate automatic slice tracking for continuous visualization of the catheter tip during navigation5. This study aims to investigate real-time automatic catheter balloon tracking using a deep-learning-based approach.Methods
Proposed MethodAll imaging was performed using a 1.5T scanner (MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany). A Residual neural network (ResNet-34)6, pre-trained using the ImageNet dataset, was employed for binary classification of the catheter balloon and background in all acquired images. Data augmentation, including random changes to brightness, contrast and Gaussian noise were implemented. The sigmoid activation function and the Sørensen-Dice coefficient loss functions were used during training, with early stopping implemented. Training and validation data (80/20) were generated from real-time single shot bSSFP images acquired in n=12 patients (8M/4F, age=40±15 years) undergoing clinical cardiac magnetic resonance examination (i.e. without a catheter present) with the following imaging parameters: TE/TR=1.25/2.5ms, Flip angle=69°, FOV=400×400mm2, voxel size=1.6×1.6mm2, slice thickness=10mm, BW=1002Hz/px, GRAPPA factor=2, no. slices=60 (20 in each transverse, sagittal and coronal orientation). Artificial catheter signal was then retrospectively placed at various locations in the cardiovascular anatomy and modelled as a 2D Gaussian-shaped signal (standard deviation in both spatial dimension=2 pixels, amplitude=maximal signal intensity of the underlying image).
Evaluation
The proposed network was evaluated on a total of 720 test images acquired from 4 consecutive patients (4M, age=10±2 years) who underwent a right heart MRI-guided cardiac catheterisation procedure at our institution. Imaging parameters, similar to the aforementioned parameters, were employed. A partial saturation (pSAT) pulse (pSAT angle=30-70°)7 was used for the simultaneous visualisation of a gadolinium-filled balloon-wedge catheter and surrounding anatomy. The proposed ResNet-34 network was evaluated with a binary classification confusion matrix to assess the robustness of detection/no detection of catheter signal. A correct detection (i.e. true positive) here was considered visually as a single segmented region intersecting the true region of the balloon signal, else classified as an incorrect detection (i.e. false positive). If more than one region was detected this was also classified as an incorrect detection. A correct “no detection” classification (i.e. zero output) was classified as a true negative. If the neural network completely failed to output a mask that segments true catheter signal this was considered a false negative. Training and testing of the network were performed using a SCAN workstation (AMD Ryzen 9 5950X 16-Core Processor running at 3.40 GHz, 128 GB of RAM, NVIDIA RTX A6000 GPU).
Results
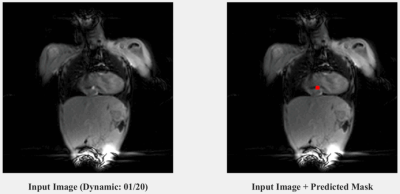
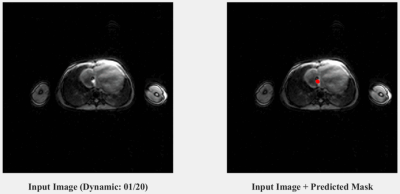
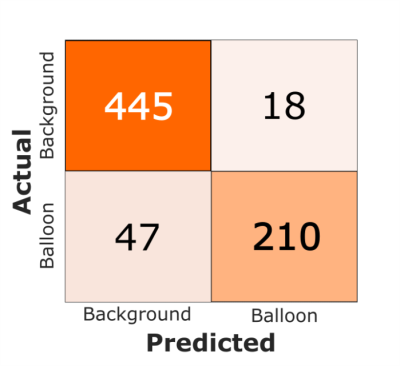
An example prediction on an artificially generated input (i.e. one with a retrospectively placed catheter signal from the validation set) after training ResNet-34 is shown in Figure 1. Two dynamic examples from the patient (i.e. test) dataset (one coronal and one transverse) are shown in Figures 2 and 3 respectively, highlighting the accurate predictions of the network at locating real catheter signal. The computation time of the network prediction (actual segmentation) is ~10 milliseconds per image. The corresponding confusion matrix evaluating the trained network is shown in Figure 4, where the sensitivity, specificity and accuracy of the network were evaluated to be 82%, 96% and 91%, respectively.Discussion
The proposed deep-learning-based approach for automatic catheter tip localisation for real-time interventional cardiac magnetic resonance imaging is feasible and accurate. It uses a neural network trained with images containing artificially generated catheter signal. Large training datasets can efficiently be generated, reducing the time-consuming process of manually segmenting catheter signal on real data. The fast computation time to detect and segment catheter signal enables application under real-time conditions. In-line implementation is now needed to study the potential benefit of the technique in enhancing the guidance of catheterisation procedures. A limitation of this study is the small patient cohort and limited imaging plane orientations used in this study. This warrants evaluation under a more diverse set of patients, which would also include various acquired image orientations.CONCLUSION
Large image training datasets with artificially generated catheter signal was successfully used to train a deep neural network for accurately detecting and segmenting catheter balloon signal, which is compatible with real-time conditions. Further investigations, with in-line integration and a larger patient cohort, are now warranted to determine the benefit of this technique for improved catheter navigation.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the EPSRC Doctoral Training Partnership (DTP) grant (EP/R513064/1), the British Heart Foundation (BHF) (PG/19/11/34243 and PG/21/10539), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, and Siemens Healthineers. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Razavi R, Hill D L G, Keevil S F, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. The Lancet. 2003;362(9399):1877-1882.
2. Rogers T, Ratnayaka K, Khan J M, et al. CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Reson. 2017;19(1):54.
3. Muthurangu V, Taylor A, Andriantsimiavona R, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Circulation. 2004;110(7):826-834.
4. Forte M N V, Roujol S, Ruijsink B, et al. MRI for Guided Right and Left Heart Cardiac Catheterization: A Prospective Study in Congenital Heart Disease. 2020:53(5):1446-1457.
5. Shankar R.V, Neji R, Duong P, et al. Automatic Tip Tracking of Gadolinium-filled Balloon Wedge Catheter during MR-guided Cardiac Catheterization. Proceedings of the International Society for Magnetic Resonance in Medicine (Montreal, Canada). 2019:P3844.
6. He K, Zhang X and Sun J. Deep Residual Learning for Image Recognition. arXiv. 2015: 1512.03385.
7. Forte M N V, Pushparajah K, Schaeffter T, et al. Improved passive catheter tracking with positive contrast for CMR-guided cardiac catheterization using partial saturation (pSAT). J Cardiovasc Magn Reson. 2017;19:60.
Figures