4970
Real-time Automatic Passive Catheter Tracking During MR-guided Cardiac Catheterization1Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Camberley, United Kingdom, 3Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
Synopsis
Keywords: Heart, MR-Guided Interventions, Tracking, Real-time
MR-guided cardiac catheterization procedures currently employ passive tracking approaches to follow the gadolinium-filled catheter balloon during catheter navigation. This requires frequent manual tracking and repositioning of the imaging slice, especially when the catheter moves out-of-plane during the procedure. In this study, we developed a novel MRI guidance approach that enables automatic real-time tracking of the catheter balloon and repositioning of the imaging slice for continuous visualization of the balloon during catheter navigation. We first demonstrate the proposed approach in a phantom and subsequently present an initial evaluation in patients.Introduction
MRI is an attractive alternative to X-ray fluoroscopy for the guidance of cardiac catheterization procedures due to high soft tissue contrast, superior hemodynamic data, and lack of ionizing radiation.1–3 Current passive tracking approaches using gadolinium (Gd)-filled balloon wedge catheters require frequent manual slice tracking and manipulation of the imaging plane to follow the catheter during catheter navigation. It was previously reported that the catheter was out-of-plane in >30% of dynamic frames during navigation.4 In this study, we present further technical developments to a novel cardiac MRI sequence5 that enables automatic real-time tracking and visualization of the balloon during catheter navigation. The proposed technique is first evaluated in a phantom and subsequently demonstrated in patients undergoing MR-guided cardiac catheterization.Methods
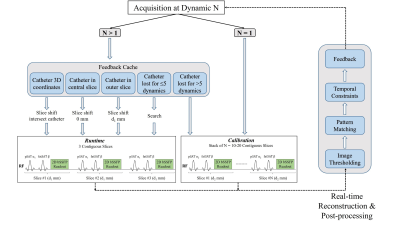
(1) Framework: The proposed prototype sequence5 consists of two imaging modes: Calibration and Runtime (Figure 1). For optimal/improved contrast between the balloon and cardiovascular anatomy, non-selective partial saturation (pSAT)6 and fat suppression pulses are used. The sequence starts with the Calibration mode where a fixed stack of contiguous slices (n=10–20, 10 mm thickness, pSAT=90°) is acquired in <2–3 s. Real-time post-processing of this slice stack using intensity thresholding and pattern matching is performed within the prescribed shim box to compute the initial 3D coordinates of the balloon. The sequence then automatically switches to the Runtime mode where three contiguous slices in the orientation of interest (10 mm thickness, pSAT=30–50°) are acquired continuously. Initially, these three slices are automatically adjusted based on the 3D coordinates obtained from the Calibration mode to intersect the balloon in the central slice. During Runtime, the balloon position is continuously estimated from real-time post-processing of the three slices using intensity thresholding, pattern matching, and spatiotemporal constraints. Post-processing is restricted to the volume of interest covered by the prescribed shim box to exclude hyperintense regions like fat or interfaces and avoid false identification of structures/spots. If the balloon is detected in one of the outer slices, the three slices are repositioned to ensure that the catheter is in the central slice. Furthermore, the sequence switches back to the Calibration mode if the balloon is lost for >5 dynamics, for e.g. due to a sudden leap of the catheter beyond the three-slice through-plane range in the Runtime mode.(2) Experimental evaluation: The proposed framework was evaluated in a 3D printed heart phantom and in two patients undergoing MR-guided cardiac catheterization. All imaging experiments were performed on a 1.5T MRI scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). The balloon of the wedge catheter was filled with 1% gadolinium (Dotarem®) for positive contrast visualization. The 2D single-shot bSSFP acquisition parameters were: TR/TE=2.44/1.02 ms, FA=50°, FOV=450×450 mm2, resolution=1.4×1.4 mm2, slice thickness=10 mm, bandwidth=1010 Hz/px, GRAPPA factor=2, partial Fourier=5/8. The Calibration stack was prescribed in the coronal orientation for fast screening of the cardiovascular system and remained fixed during the procedure. The Runtime slices can be prescribed freely in the orientation of interest for optimal visualization of the desired structures.
Results
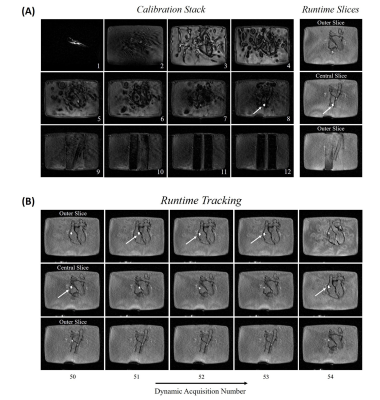
Figure 2(A) shows still frames of the phantom experiment with the balloon successfully detected in the Calibration stack (slice 8) followed by the three Runtime slices correctly centred on the balloon. Figure 2(B) shows the Runtime slices acquired at later dynamics demonstrating automatic slice repositioning to follow the balloon in the central slice. Figure 3 shows an animation where the catheter was continuously tracked while it was navigated through the phantom. Figures 4 (still frames) and 5 (animation) demonstrate the application of the proposed approach in one patient. As before, the balloon was initially detected in the Calibration mode and then tracked with slice repositioning during Runtime while the catheter was manipulated. In the Calibration mode, the balloon was automatically identified with 100% accuracy both in the phantom and in vivo. In the Runtime mode, the detection accuracy was 94.8% (88.5% in the central slice) in the phantom and 77.6±23.8% (59.1±2.8% in the central slice) in vivo.Discussion
The proposed sequence can provide a more robust guidance approach as it avoids manual slice repositioning and manipulation of the imaging plane during catheter navigation. This sequence can also facilitate the fast detection of out-of-plane catheters, which are time-consuming to locate when manually tracked and may prolong the procedure. A current limitation of the proposed sequence is decreased temporal resolution due to the acquisition of three contiguous slices in the Runtime mode. Future work would focus on implementing acceleration techniques to achieve higher framerates and performing a larger clinical study to establish its advantage over existing techniques.Conclusion
The proposed guidance approach enables continuous and robust automated slice repositioning and catheter tracking during MR-guided cardiac catheterization.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the British Heart Foundation (BHF) grants (PG/19/11/34243 and PG/21/10539), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, and Siemens Healthineers. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] Razavi R, Hill DL, Keevil SF, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362(9399):1877-1882.
[2] Pushparajah K, Chubb H, Razavi R. MR-guided Cardiac Interventions. Top Magn Reson Imag. 2018;27(3):115-128.
[3] Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34(5):380-389.
[4] Velasco Forte MN, Roujol S, Ruijsink B, et al. MRI for Guided Right and Left Heart Cardiac Catheterization: A Prospective Study in Congenital Heart Disease. J Magn Reson Imag. 2020:53(5):1446-1457.
[5] Shankar RV, Neji R, Duong P, et al. Real-time Automatic Tip Tracking of Gadolinium-filled Balloon Wedge Catheter during MR-guided Cardiac Catheterization. Proc Intl Soc Magn Reson Med 27. Montreal (QC), Canada. 11-16 May 2019. P3844.
[6] Velasco Forte MN, Pushparajah K, Schaeffter T, et al. Improved passive catheter tracking with positive contrast for CMR-guided cardiac catheterization using partial saturation (pSAT). J Cardiovasc Magn Reson. 2017;19(1):60.
Figures