4969
Cardiac MRI at 3.0T in the presence of implantable cardioverter defibrillators (ICDs)1Medicine I, University Hospital Wuerzburg, Wuerzburg, Germany, 2Philips GmbH, Hamburg, Germany, 3Biotronik SE Co.KG, Berlin, Germany
Synopsis
Keywords: Myocardium, Visualization
Performing a cardiac MRI in the presence of active cardiac implants remains a challenge due to the extensive artifact burden caused by the implant. Sequence solutions established at 1.5 T have yet to be transferred to a clinical use at 3T. In this work, we use phantom and volunteer measurements in order to establish an adapted 3T protocol for CINE with TFE and wideband LGE-PSIR sequences that sufficiently suppress image artifacts caused by active implants. The first clinical data show the feasibility of this protocol and its ability to detect scar tissue in patients with implanted ICD.Introduction
Technical advances allow the secure examination of patients with MRI-conditional active cardiac implants with MRI even at field strengths of 3.0T (1). Imaging at 3.0T offers higher signal-to-noise ratios than at 1.5T, but the artifact burden increases as well, obscuring the potential benefit of a higher field strength. The type of active cardiac implants affect artifact burden, with ICDs causing the largest burden (2, 3). However, particularly patients with ICDs often require a reassessment of myocardial scar and fibrosis distribution due to increased ventricular arrhythmias or failing heart function (4) and would thus profit from artifact-free MR images. At 1.5T, changes in the imaging protocols such avoidance of bSSFP cine sequences have been proven useful. Wideband LGE imaging has been established for artifact-free scar and fibrosis detection with in vivo studies highlighting the usefulness of cardiac MRI prior to ablation therapies (5). Data on the implementation of such techniques to the setting of 3.0T are scarce and limited to in vitro and animal measurements (6). In this work, we aim to establish a modified cardiac imaging protocol including wideband LGE for patients with implanted active implants at 3.0T and to perform this modified protocol on such patients.Methods
All measurements were performed on a clinical 3T MRI scanner with commercial anterior and posterior body surface coils for signal reception (AchievaDS, dStream Whole body, Philips Healthcare). For the phantom measurements, an in-house built cubic phantom with an implemented spherical grid system was used. Tubes with short T1 times were mounted within the grip system in order to evaluate the efficacy of the inversion pulses. For LGE imaging, broadband adiabatic inversion pulses (TR 3ms, TE 1 ms, flip angle 15°, echo train length 49) with bandwidths of 1kHz, 2kHz, 3kHz and 4 kHz, and frequency offsets from -1000 Hz to +1500 Hz in steps of 500 Hz were tested. CINE Imaging included bSSFP and TFE sequences with partial echo and optional flow compensation. Representative ICDs (Ilesto 7 HF-T; Activor 7 HF-T QP, Biotronik) were used during the phantom measurements. In vivo measurements included 7 healthy volunteers and 4 patients scheduled for an CMR with LGE imaging. An ICD was taped externally to the upper left chest of these participants. Additionally, 3 male patients with an ICD (two implanted on the left upper (Rivacor 5VR-T, Iforia 7VR-T), one on the right upper pectoral region (Rivacor 5D-RT)) were examined using the previously established sequences. One patient with severe ischemic heart disease was referred due to recurrent VTs prior to ablation therapy, and two patients with non-ischemic heart disease were referred for reassessment of scar/fibrosis burden.Results
The phantom measurements without positioned implant show the effects of different bandwidth and frequency offsets on the signal intensities of the spherical grip system and the efficacy of signal suppression within the small tubes with short T1. Depending on the bandwidth, ripple artifacts can be present. In the presence of an ICD, the comparison of different bandwidths offsets show that the best results are obtained with a bandwidth of 3 kHz and an offset of +750 Hz (Fig 1). The volunteer measurements showed that a spoiled TFE sequence without flow compensation and partial echo resulted in in an overall good image quality allowing clinical interpretation and evaluation (Fig. 2, 3). The comparison of CINE imaging with the modified TFE sequences prior and after administration of contrast agent showed an improved image quality of the TFE sequence after contrast agent administration. However, in cases with no visual artifacts within the cardiac region, the bSSFP CINE imaging retains its applicability (Fig.4). Wideband PSIR-LGE with a bandwidth of 3kHz and an offset of 750 Hz for the inversion pulse allowed sufficient suppression of hyperintense frequency offset artifacts and a clear delineation of scar and fibrosis areas, even in small structures such as the papillary muscle.Discussion
Overcoming the artifact burden caused by active cardiac implants is a prerequisite for the clinical application of cardiac MRI at 3.0T for patients with such implants. The phantom measurements demonstrate the different artifact qualities such as central signal void, hyperintense areas in the vicinity of the implant, and geometric distortions. The results also show that additional artifacts such as rippling can be induced by the adapted image sequences. The CINE imaging with TFE CINE allows the clinical analysis of wall motion and volumetry; however, the sequences should best be run after the application of contrast agents for improved contrast between myocardium and blood. The modified protocol for scar detection with a wideband inversion pulse at a bandwidth of 3kHz and an offset of 750 Hz allows a reasonable compromise between homogeneous signal intensities and good suppression of the short T1 test tubes and the artifact burden.Conclusion
The presented work shows the successful implementation of TFE CINE and wideband PSIR-LGE for cardiac imaging of patients with ICDs at 3.0T.Acknowledgements
We thank I. Perdijk for her help in performing the measurements.References
1. Sommer T, Bauer W, Fischbach K, Kolb C, Luechinger R, Wiegand U, et al. MR Imaging in Patients with Cardiac Pacemakers and Implantable Cardioverter Defibrillators. Rofo. 2017;189(3):204-17.
2. Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk R, Saliaris A, et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2014;37(10):1274-83.
3. Reiter T, Weiss I, Weber OM, Bauer WR. Signal voids of active cardiac implants at 3.0 T CMR. Sci Rep. 2022;12(1):6285.
4. Mukherjee RK, Whitaker J, Williams SE, Razavi R, O'Neill MD. Magnetic resonance imaging guidance for the optimization of ventricular tachycardia ablation. Europace. 2018;20(11):1721-32.
5. Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270(1):269-74.
6. Ranjan R, McGann CJ, Jeong EK, Hong K, Kholmovski EG, Blauer J, et al. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace. 2015;17(3):483-8.
Figures
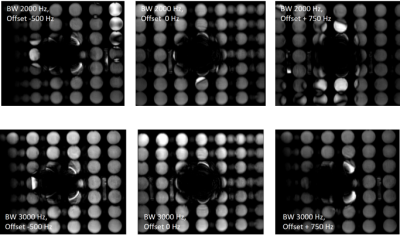
The effects of different bandwidths and offsets on artifacts induced by an ICD
The artifact burden caused by an ICD consist of a central signal void, distortion and hyperintense artifacts in the vicinity of the central signal void. At a bandwidth of 3kHz and an offset of +750 Hz, the signal of the spherical grid appears the most homogeneous, and the signal of the test tubes with short T1 is nearly completely suppressed. As a trade-off, at the bandwidth of 3 kHz, the central signal void increases with the offset, and it ranges from 11x6 cm at an offset of -500 Hz to 12x9 cm at an offset of 750 Hz.
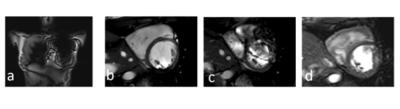
Volunteer measurement.
A 30yo female volunteer with an externally placed ICD on her left upper pectoral region (a) was examined prior (b) and after (c, d) placement of the ICD. Without ICD, the short axis view obtained with a bSSFP is nearly artifact free (b), whereas in the presence of an ICD, the image does not meet a diagnostic quality. The same view with a TFE sequence without flow compensation and with partial echo allows suppression of most artifacts.
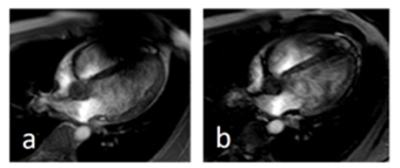
Volunteer measurement
A 24yo male volunteer with an externally placed ICD on his left upper pectoral region was examined with a TFE sequence with flow compensation and no partial echo (a) and no flow compensation and with partial echo (b). The latter sequence successfully suppresses the majority of artifact burden covering the right ventricle.
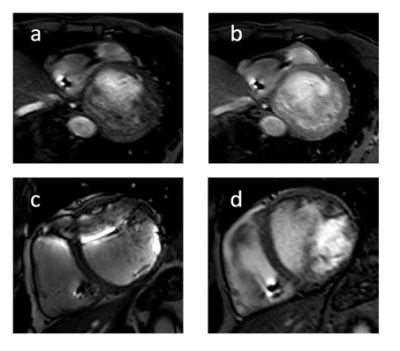
TFE imaging prior and after application of contrast agent
Patient with right sided ICD Basal short axis slide obtained with a TFE sequence prior (a) and after (b) application of contrast agent. The image contrast is blurred on the left side, and the endomyocardial border is obscured by flow artifacts. After application of contrast agent, the endomyoardial boarder is clearly depicted. Patient with a left sided ICD. The TFE sequence (d) shows more intracavitary flow artifact compared to the bSSFP imaging (c) but allows even the visualization of the anterior myocardial wall.
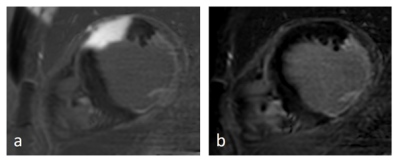
Wideband LGE-PSIR imaging
Scar imaging in a patient with ischemic heart disease and implanted ICD (left side). The unmodified PSIR-LGE (a) causes a hyperintense artifact completely obscuring the view of the anterior wall. The wideband PSIR-LGE allows complete suppression of this artifact and clear depiction of the near-transmural scar and fibrosis of the posterior papillary muscle.