4959
Accelerating Multi-Contrast Imaging Near Metallic Implants with Variable Resolution Sampling and Joint Reconstruction1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Image Reconstruction, Image Reconstruction
In multi-spectral imaging near metallic implants, redundant information is present in neighboring spectral bins and contrasts due to the overlapping and smooth spectral profiles. This redundancy is exploited here to reduce scan time when multiple image contrasts are desired.Introduction
Multi-spectral imaging (MSI) near metal demands long scan times owing to the need to acquire several interleaved fast spin echo (FSE) volumes[1,2,3]. Here, it is demonstrated that the scan time can be nearly cut in half when complementary information between clinically desirable contrasts and overlapping spectral profiles are leveraged.Methods
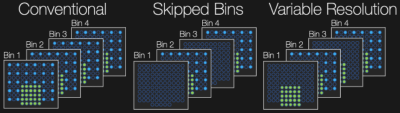
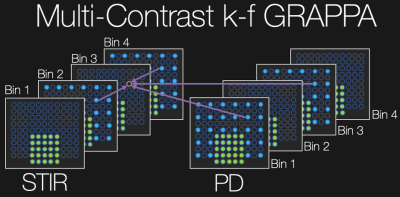
MSI acquisitions consist of interleaved 3D FSE acquisitions, often in multiple passes or concatenations to reduce crosstalk while achieving the desired TR. A variable resolution (VR) undersampling scheme as depicted in Figure 1 was implemented in which all parallel imaging/partial Fourier-accelerated k-space data are acquired for even spectral bins while the odd bins contain only the autocalibration signal (ACS) region. When two contrasts are desired, the undersampling occurs in complementary bins such that one bin contains all k-space points for at least one contrast.A linear multi-contrast k-frequency (MC k-f) GRAPPA kernel was estimated via the ACS region to interpolate the higher spatial frequency k-space points in contrasts/bins wherein only the ACS data were acquired (as depicted in Figure 2). Conventional 2D GRAPPA[4] was employed within each contrast/bin followed by a projection onto convex sets partial Fourier reconstruction.
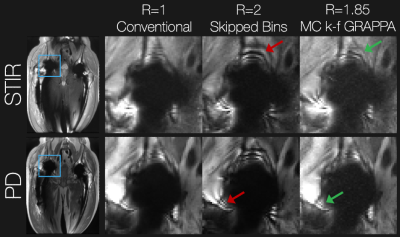
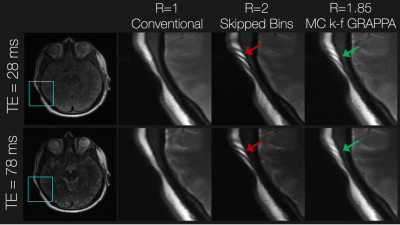
These methods were assessed with a retrospective undersampling experiment. Proton density (PD) and short-tau inversion recovery (STIR) PD MSI data with 24 spectral bins (2.25 kHz bandwidth and 1 kHz spacing) were collected at 3T in a consenting subject having bilateral total hip replacements. The total acquisition time for the PD scan (TE/TR = 8.3/3000 ms) was 4 min 54 seconds, while that of the STIR-PD scan (TE/TR/TI = 7.8/5500/175 ms) was 5 min and 19 seconds. Measurements were made using 55 RF coil channels. Similarly, this method was assessed for accelerated PD/T2-weighted (TR=5000 ms and TE = 28/78 ms, respectively) in the brain of a subject with a piece of cobalt chromium metal placed near their skull.
Root-sum-of-squares bin-combined images were generated from spectrally fully sampled data, the proposed approach, and from naively omitting every other bin from the reconstruction.
Results
Figure 3 shows the results in the total hip replacement subject. Compared with the conventional approach, skipping spectral bins results in severe ripple artifacts (red arrows) arising from incomplete spectral coverage, which are mitigated when using the VR sampling scheme and joint multi-contrast k-f GRAPPA. Contrast and spectral coverage are maintained at the expense of apparent g-factor noise amplification. Similar results were observed in the brain experiment shown in Figure 4. The proposed approach mitigated artifacts from incomplete spectral coverage compared with the skipped-bin approach.Discussion
At the expense of g-factor noise amplification, the amount of data acquired in each spectral bin and image contrast can be modulated according to the proposed variable resolution sampling scheme, and a joint multi-contrast multi-frequency image reconstruction algorithm can recover artifact-reduced imaging volumes. Future work will optimize the GRAPPA kernel geometry to minimize the noise amplification, and applications in higher resolution MSI will be pursued.Acknowledgements
This research was supported by National Institute of Health (NIH) grant R21EB030123.References
[1] Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009 Feb;61(2):381-90.
[2] Lu, W., Pauly, K. B., Gold, G. E., Pauly, J. M., & Hargreaves, B. A.. SEMAC: slice encoding for metal artifact correction in MRI. Mag Reson Med,2009, 62(1), 66-76.
[3] Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, McKinnon GC, Potter HG, King KF. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011 Jan;65(1):71-82.
[4] Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002 Jun;47(6):1202-10.
Figures