4958
Retrospective and prospective accelerated T1ρ/T2 mapping with Compressed Sensing: high resolution T1ρ mapping and simultaneous T1ρ/T2 mapping1Department of Biomedical Engineering, Program of Advanced Musculoskeletal Imaging (PAMI), Cleveland Clinic, Cleveland, OH, United States, 2Department of Electrical, Computer, and Systems Engineering, Case Western Reserve University, Cleveland, OH, United States, 3Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 4Electrical Engineering, University at Buffalo, State University of New York, Buffalo, NY, United States, 5Department of Diagnostic Radiology, Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 6Biomedical Engineering, University at Buffalo, State University of New York, Buffalo, NY, United States
Synopsis
Keywords: Image Reconstruction, Cartilage
Quantitative MR T1rho T2 imaging shows promising results on detecting early-stage osteoarthritis, but long scan time limits the spatial resolution, making it vulnerable to partial volume averaging. Such effect reduces the sensitivity to small focal degeneration. In this study, compressed sensing reconstruction with spatio-temporal finite difference regularization was used to accelerate high-resolution (slice thickness < 2mm) T1rho imaging and standard-resolution simultaneous T1rho and T2 imaging, and evaluated the result comparing with reference imaging, retrospective and prospective reconstruction, and scan-rescan repeatability.Introduction
Osteoarthritis (OA) is the most common type of arthritis which affect various tissues of the joint and afflicts more than 30 million people in US alone. Among various MRI techniques, quantitative imaging techniques such as T1ρ and T2 have shown correlation to glycosaminoglycans (GAG) concentration and collagen structure in cartilage, which is known to alter in the early stage of OA.1,2 However, current T1ρ and T2 mapping in human subjects are primarily limited to relatively low resolution (0.6-1mm in-plane and 3-5mm slice thickness), limiting the sensitivity to small focal degeneration due to partial-volume averaging. The recent advancements in image reconstruction such as compressed sensing (CS) and deep learning allowed higher acceleration compared to parallel imaging reconstruction.3,4 Such techniques can potentially enable acquisition of high-resolution T1ρ and T2 mapping (slice thickness < 2mm), which however has not been evaluated in human subjects yet. In this study, CS acceleration was applied for two-fold goals: first, to allow acquisition of T1ρ mapping with much higher resolution as compared to the current standard resolution; second, to allow simultaneous acquisition of T1ρ and T2 mapping with standard resolution with a shorter time. All acquisitions were compared with GRAPPA 2 accelerated reference acquisition, with retrospective and prospective CS reconstruction, and in terms of scan-rescan repeatability.Methods
Data AcquisitionTotal six volunteers were scanned using 3T Prisma scanner (Siemens Healthcare, Erlangen). 3D MAPSS5,6 was used to collect T1ρ and T2 mapping with two different setups, high-resolution (0.36*0.73*1.6mm) 8-echo T1ρ acquisitions and standard-resolution (0.44*0.88*4mm) 7-echo T1ρ/T2 combined acquisition (T1ρ and T2 share the first echo), both using GRAPPA 2 for reference scan (as noted in Table 1, the reference scan for high-resolution T1ρ mapping is more than 30 mins with GRAPPA 2). Prospectively-undersampled data were acquired with different acceleration factors (AFs). The Dual-echo steady-state (DESS) images were collected for cartilage segmentation. Patients were scanned with the same sequence except for high-resolution reference scan after fully coming down from the table for scan-rescan repeatability. Detailed scan parameters can be found in Table 1.
Compressed Sensing Reconstruction
Reference quantitative scan was reconstructed with GRAPPA reconstruction, and multi-coil images were combined with complex coil-combination. CS reconstruction was applied to retrospectively-undersampled k-space from reference and prospectively-undersampled k-space. Monotone fast iterative shrinkage/thresholding algorithm (MFISTA) combined with the fast gradient projection (FGP) algorithm was used with spatiotemporal finite difference (STFD) regularization.4 For combined T1ρ/T2, the echoes were reordered so the signal curve is monotonically decreasing.
Quantitative Evaluation
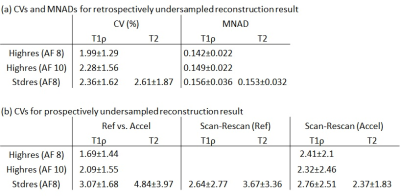
After reconstruction, DESS images were registered to the first echo of the reference images to create 6 compartment cartilage segmentations (Lateral/Medial Femoral Cartilage (LFC/MFC), Lateral/Medial Tibial Cartilage (LT/MT), Trochlear (TRO), and Patellar (PAT) cartilage). The reference undersampled images were fitted with non-linear least-squares (NLLS) algorithm. Pixel-wise median normalized absolute difference (MNAD) and cartilage compartment-wise mean value difference in terms of coefficient of variation (CV) was calculated between the reference and retrospectively-accelerated maps. Cartilage compartment-wise CVs was calculated between reference and prospectively-accelerated maps and scan-rescan for repeatability.
Results
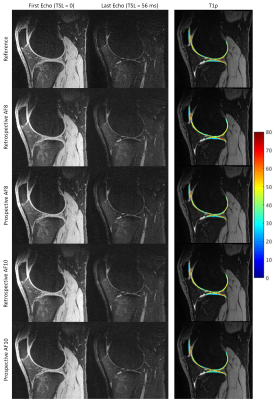
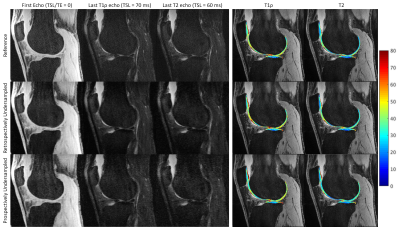
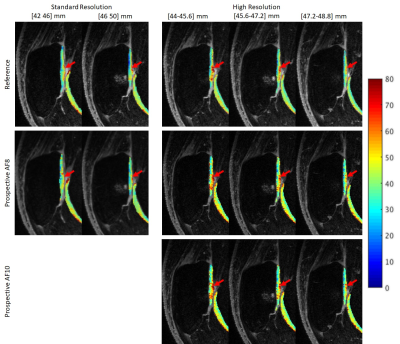
Figure 1-3 show example images of retrospectively- and prospectively-undersampled images with their respective reference. All high-resolution scans had at least 10 average SNR in the last echo. The STFD regularization introduces some blurring in image, but no significant degradation is present and fine details are preserved.Table 2 shows CVs and MNADs calculated from retrospectively- and prospectively-undersampled reconstruction. Pixel-wise MNADs calculated by retrospective data showed smaller values in high-resolution compared to standard-resolution. In general, CVs between reference and accelerated was larger for prospective data compared to retrospective data, but all showed small difference within 5%. All acquisitions showed excellent scan-rescan repeatability, and standard-resolution accelerated scans showed comparable or better repeatability compared to standard-resolution reference scans.
Discussion
The accelerated scans could allow simultaneous T1ρ and T2 acquisition with standard resolution within less than 3 minutes, and an 8-echo high-resolution T1ρ acquisition in less than 7 minutes without significant degradation in image quality or quantitative accuracy. For high-resolution mapping, we used T1ρ as an example, but similar results are expected for T2 mapping. High-resolution maps will significantly improve diagnostic capability. The reduced scan time can also greatly reduce the motion artifact and improve patient comfort as well as throughput. The high-resolution acquisition showed better reconstruction results in all metrics compared to standard-resolution acquisition even with lower SNR. Also, the smoothing effect of the STFD regularization used in CS reconstruction was more prominent in standard-resolution acquisition, whereas the accelerated acquisition with high-resolution visually appeals compared to the reference scan with some denoising effect and without noticeable smoothing effects in reconstructed images. These suggest that the CS is more favorable for higher resolution. However, more sophisticated comparisons such as preservation of image features should be evaluated. Also, the regularization factor of STFD regularization changes the quantification outcome due to its nature of regularizing temporal difference, so other regularization methods robust to exponential decay should be investigated.Conclusion
Acceleration with compressed sensing reconstruction allows high-resolution relaxation time mapping with reasonable scan time (7 mins) and allows simultaneous T1ρ and T2 mapping with standard-resolution in a very short time (<3 mins) without degradation compared to reference acquisition and with excellent scan-rescan repeatability.Acknowledgements
This study was supported by NIH/NIAMS R01 AR077452References
1. Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, Thiessen JD, Thompson RT. MRI T2 and T1rho relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC musculoskeletal disorders. 2019;20(1):182.
2. MacKay JW, Low SBL, Smith TO, Toms AP, McCaskie AW, Gilbert FJ. Systematic review and meta-analysis of the reliability and discriminative validity of cartilage compositional MRI in knee osteoarthritis. Osteoarthritis Cartilage. 2018;26(9):1140-52.
3. Zhou Y, Pandit P, Pedoia V, Rivoire J, Wang Y, Liang D, et al. Accelerating T1rho cartilage imaging using compressed sensing with iterative locally adapted support detection and JSENSE. Magnetic resonance in medicine. 2016;75(4):1617-29
4. Zibetti MVW, Sharafi A, Otazo R, Regatte RR. Accelerating 3D-T1rho mapping of cartilage using compressed sensing with different sparse and low rank models. Magnetic resonance in medicine. 2018;80(4):1475-91.
5. Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magnetic resonance in medicine. 2008;59(2):298-307.
6. Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, et al. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis and cartilage. 2015;23(12):2214-23.
Figures