4943
Modulation of left anterior cingulate glutathione in Lithium treatment of bipolar disorder1Cleveland Clinic Foundation, CLEVELAND, OH, United States, 2Brigham and Women's Hospital, Boston, MA, United States
Synopsis
Keywords: Psychiatric Disorders, Spectroscopy
Changes in total glutathione (GSH) level in left anterior cingulate cortex (ACC) following lithium monotherapy of bipolar disorder in depressed state were studied at7T. Patients were scanned with a semi-LASER sequence at baseline and 2, 8 and 26 weeks from onset of therapy. Healthy controls were also scanned at those 4 time points. An increase in GSH level at left dorsal/rostral ACC was observed in patients, and the increase took place between 8 and 26 weeks after the start of therapy.Introduction
Bipolar disorder (BD) is a condition characterized by periods of uncharacteristically elevated mood followed by periods of depression.[1,2] Psychopathology of BD in animal models has been associated with cellular oxidative stress.[3,4] Glutathione (GSH) has antioxidant action and reduces reactive oxygen species (ROS).[5] Increase in ROS and increase in oxidative stress is caused by reduction in GSH.[5] BD has also been linked to plasma GSH reduction.[4]Investigation of GSH modulation at anterior cingulate cortex (ACC) is important because of its involvement in the pathophysiology of BD.[6-8] Despite of that, no depletion in ACC GSH were observed in euthymic BD[9] or in young people with BD.[10] Reduced ACC GSH has been linked to tobacco and alcohol use in depressed, manic, euthymic and mixed state BD[11].
Lithium (Li), having antioxidant properties, is a commonly prescribed mood stabilizing drug in BD,[12] and is reported to have no effect on dorsal ACC GSH level in euthymic BD.[9] However, dose-dependent elevation has been reported in cortical cells in rats.[13]
There is a dearth of studies exploring ACC GSH level in medication-free patients with BD in depressed state, which can explore the role of oxidative stress in BD; an inverse relationship between ACC GSH level and depression scores was reported in this group of patients.[14] Here, we have investigated modulation of left dorsal/rostral ACC GSH level following 26 weeks of Li therapy in medication-free patients with BD in depressed state.[14]
Methods
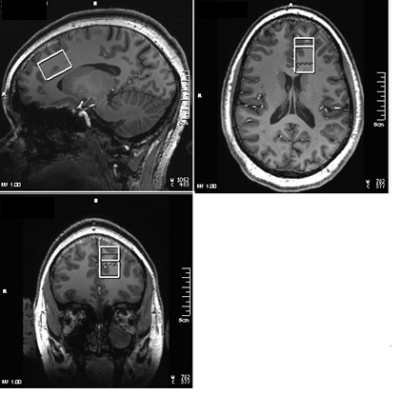
Seventeen patients with BD (30±9 y, 3 M, fulfilling Diagnostic and Statistical Manual 5th edition (DSM-V) criteria for BD with current depressive episode, 15<Hamilton Depression Rating Scale (HAM-D) score < 25, Young Mania Rating Scale (YMRS) < 8, at least 2 weeks without any psychotropic (medication free for 5 weeks in case previously on fluoxetine), no Li therapy for past 6 months, 1 week without alcohol consumption) and 9 healthy controls (29±10 y, 3 M) were scanned at Siemens 7T Magnetom scanner with a 32-channel receive head coil under an IRB-approved protocol. Subjects were scanned at baseline (pre-therapy), and after 2, 8 and 26 weeks of Li monotherapy (started at 300 mg po bid and increased aiming for a blood level of 0.6 meq/l or as tolerated). MRI scans for each subject consisted of (i) localizer, T1-weighted Magnetization Prepared Rapid Acquisition with Gradient Echo anatomical scan (TR/TE=2250/2.97ms, matrix=256×256, FOV=204×204mm2, scan time 4-min 38-s) and (iii) semi-LASER (sLASER)[15] scan with Variable Power and Optimized Relaxation Delays for water suppression[16] (TR/TE1/TE2/TE3=8000/9/11/9ms, 32 transients, scan time 4-min 24-s) scan of a 20×30×20mm3 voxel at left dorsal/rostral ACC (Fig. 1). In addition to these, water reference acquisition (with RF off) for eddy current correction and unsuppressed water signal acquisition for quantification were performed. MRspa software package (https://www.cmrr.umn.edu/downloads/mrspa/) was used to analyze sLASER data, which consisted of (i) Eddy current correction, (ii) frequency correction, (iii) phase correction, (iv) signal averaging, and (v) quantification using LCModel fitting after applying correction for tissue voxel composition. Voxel segmentation was performed using BET and FAST algorithm[17] of FSL software library.[18] Data with >20% CRLB of GSH fit were not used in analysis.Mean ACC GSH levels of patients and controls at baseline were performed using unpaired t-test. Longitudinal GSH data were analyzed using a linear mixed-effects model, which includes visit (4 time-points), group (patients and controls) and their interaction as fixed effects. The model also contains a Gaussian random intercept at the participant level. Linear contrasts were applied to estimate the changes in GSH level at different visits and their difference. Statistical significance was established at two-sided P-value<0.05. %Coefficient of variation (%CV) of GSH levels over 4 weeks was also determined for the healthy controls considering data from 6 subjects with all 4 time-points.
Results and Discussion
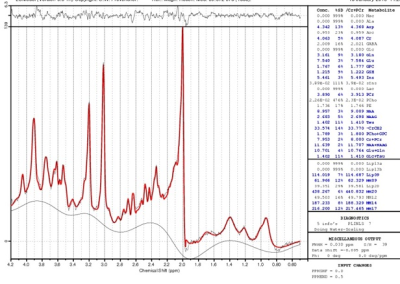
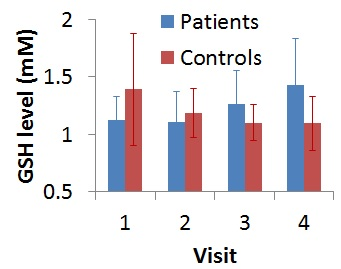
A sample LCModel fitted spectrum is shown in Fig. 2. Two patients dropped out after 2 visits, and 3 patients and 1 control dropped out after 3 visits. After accounting for data with poor fit, baseline GSH level in patients (N=14; 1.12±0.20 mM) showed a tendency to be lower than that in controls (N=8; 1.39±0.49 mM; P=0.08). A tendency towards inverse correlation between GSH levels and HAM-D scores were observed at baseline (r=-0.5094, P=0.06). %CV of GSH for the healthy controls was determined to be 11.20%. Evolution of GSH levels over the 4 visits (at baseline, 2-, 8- and 26-week) for patients and controls are shown in Fig. 3. GSH levels in patients did not show any significant change during 1st 8 weeks of Li therapy and increased significantly (P=0.03) at between 8 and 26 weeks. In addition, there was significant difference in changes in GSH level (from baseline) to 8- and 26-week between patients and controls (P=0.024 and 0.003 respectively). Despite of small sample size of this preliminary study, the results addressing depressed state of BD, is in agreement with the animal model of increase in GSH with Li treatment of BD, suggesting neuroprotective effect of GSH.[13]Conclusion
Li monotherapy increases GSH level at left dorsal/rostral ACC in otherwise medication-free patients with BD in depressed state and the increase takes place between 8 and 26 weeks after onset of therapy.Acknowledgements
This work was supported, in part, by the National Institutes of Health. We thank Sineyob Ahn, Siemens Healthineers, for support with sLASER sequence used in this study.References
[1] Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ 2012;345:e8508.
[2] Association AP. Diagnostic and Statistical Manual of Mental Disorders Arlington: American Psychiatric Publishing; 2013.
[3] Dean OM, van den Buuse M, Bush AI, Copolov DL, Ng F, Dodd S, et al. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem 2009;16:2965-76.
[4] Nucifora LG, Tanaka T, Hayes LN, Kim M, Lee BJ, Matsuda T, et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl Psychiatry 2017;7:e1215.
[5] Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489-92.
[6] Sanches M, Amorim E, Mwangi B, Zunta-Soares GB, Soares JC. Smaller left anterior cingulate cortex in non-bipolar relatives of patients with bipolar disorder. Braz J Psychiatry 2019;41:254-6.
[7] Jelen LA, King S, Horne CM, Lythgoe DJ, Young AH, Stone JM. Functional magnetic resonance spectroscopy in patients with schizophrenia and bipolar affective disorder: Glutamate dynamics in the anterior cingulate cortex during a working memory task. Eur Neuropsychopharmacol 2019;29:222-34.
[8] Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol 2001;102:373-9.
[9] Soeiro-de-Souza MG, Pastorello BF, Leite Cda C, Henning A, Moreno RA, Garcia Otaduy MC. Dorsal Anterior Cingulate Lactate and Glutathione Levels in Euthymic Bipolar I Disorder: 1H-MRS Study. Int J Neuropsychopharmacol 2016;19.
[10] Lagopoulos J, Hermens DF, Tobias-Webb J, Duffy S, Naismith SL, White D, et al. In vivo glutathione levels in young persons with bipolar disorder: a magnetic resonance spectroscopy study. J Psychiatr Res 2013;47:412-7.
[11] Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: an exploratory study. J Psychiatr Res 2014;55:59-67.
[12] Fisher E, Gillam J, Upthegrove R, Aldred S, Wood SJ. Role of magnetic resonance spectroscopy in cerebral glutathione quantification for youth mental health: A systematic review. Early Interv Psychiatry 2020;14:147-62.
[13] Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience 2007;144:1447-53.
[14] Bhattacharyya PK, Lowe MJ, Anand A. Association of anterior cingulate glutathione and degree of depression in unmedicated bipolar disorder – a 7T study. Proc Intl Soc Mag Reson Med 2021;29:1658.
[15] Scheenen TW, Heerschap A, Klomp DW. Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. MAGMA 2008;21:95-101.
[16] Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41:649-56.
[17] Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45-57.
[18] Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208-19.
Figures