4938
Prefrontal glutathione levels in major depressive disorder are linked to a loss of positive mood
Ruth O'Gorman Tuura1, Andreas Buchmann2,3, Christopher Ritter2,3, Melanie Haynes4, Ralph Noeske5, and Gregor Hasler3
1University Children's Hospital Zurich, Zurich, Switzerland, 2Center for MR Research, University Children's Hospital Zurich, Zurich, Switzerland, 3Psychiatry Research Unit, University of Fribourg, Fribourg, Switzerland, 4Translational Research Center, University Hospital of Psychiatry and Psychotherapy, Bern, Switzerland, 5GE Healthcare, Munich, Germany
1University Children's Hospital Zurich, Zurich, Switzerland, 2Center for MR Research, University Children's Hospital Zurich, Zurich, Switzerland, 3Psychiatry Research Unit, University of Fribourg, Fribourg, Switzerland, 4Translational Research Center, University Hospital of Psychiatry and Psychotherapy, Bern, Switzerland, 5GE Healthcare, Munich, Germany
Synopsis
Keywords: Psychiatric Disorders, Spectroscopy
Major depressive disorder (MDD) is one of the most common neuropsychiatric disorders, with symptoms including persistent sadness and low mood. MDD is associated with neurochemical alterations in GABA, glutamate, and glutamine levels, but to date few studies have examined changes in glutathione (GSH) in MDD. This study investigated changes in GSH in a medication naïve group of participants with current and past depression, and healthy controls, and the link between GSH, stress markers, depressive symptoms and mood. We observed elevated prefrontal GSH in participants with current but not remitted depression, which was associated with a decrease in positive mood.Introduction
Major depressive disorder (MDD) is one of the most common neuropsychiatric disorders worldwide, with an estimated lifetime prevalence of 10.8%1, increasing to 19% among adolescents2. Symptoms of MDD include a persistent sadness and low mood, anhedonia (loss of pleasure), irritability, low energy levels, altered appetite, and disrupted sleep.3 However, MDD is clinically heterogeneous, with symptoms varying between participants or within a participant over time, possibly due to differences in stress reactivity4 or inflammation5. Understanding the neurochemical changes associated with depression, and the role of stress and inflammation in MDD, is important for developing targeted and effective therapies and improving treatment response. MRS studies of depression have revealed neurochemical changes including alterations in GABA, Glutamate and Glutamine, and Choline levels6,7. However, to date few studies have examined changes in glutathione in MDD. Glutathione is a tripeptide synthesized from glutamate, cysteine, and glycine8, which acts as an antioxidant and plays an important role in cell signalling, differentiation, and proliferation, gene expression and protein function. It is also a marker for oxidative stress, which is thought to play an important role in the pathogenesis of MDD9. However, MRS studies investigating GSH changes in depression have shown mixed results, with both increases and decreases reported.8,10 The aim of this study was to investigate changes in GSH in a medication naïve group of participants with current and past depression, and healthy controls, and to investigate the link between GSH, stress markers, depressive symptoms and mood.Methods
The participant group consisted of 66 young adults (mean age 25 years, range 18-39) recruited from the community, including 20 healthy controls, 34 participants with past MDD, and 12 participants meeting the diagnostic criteria for current MDD (see table 1 for group demographics). MRI and MRS data were collected with a 3T Discovery MR750 MRI scanner, using an 8 channel head coil. GSH-edited spectra were collected from a 25x40x30 mm3 voxel centred in the left dorsolateral prefrontal cortex, with the MEGAPRESS method (TE/TR=131/1800 ms, 128 edit ON/OFF pairs), with editing pulses applied at 4.56 and 20 ppm. A 3D inversion recovery (IR) prepared spoiled gradient echo volume was also collected (TE/TR/TI = 5/11/600 ms, flip angle = 8 degrees, resolution = 1x1x1mm3) for planning the voxel position and for subsequent correction for tissue composition. Spectra were preprocessed and analysed with Gannet version 3.0, and GSH levels were calculated as water scaled concentrations. The severity of depressive symptoms was evaluated with the Beck Depression Index (BDI), the Hamilton Depression Rating Scale (HAM-D), and the Montgomery-Asberg Depression Rating Scale (MADRS). In addition, trait positive and negative affect (mood) was assessed with the Positive and Negative Affect Schedule (PANAS), and perceived stress was assessed with the perceived stress scale (PSS). Cortisol levels, as an additional marker for acute stress, were assessed from a saliva sample taken before each scan. Groupwise differences between the control, past MDD, and current MDD groups were assessed with a Kruskal-Wallis test, and post-hoc tests were performed with a median test. The link between GSH levels and symptom scales were assessed with Spearman correlations. Statistical analyses were performed with SPSS 27.0.Results
Trend-level groupwise differences in GSH were observed (p=0.074), which became significant (p=0.038, Kruskal-Wallis test) after removal of two outliers (one control and one participant with past MDD) with GSH levels more than four times the group median values. Post-hoc comparisons showed elevated GSH levels in the participants with current depression (figure 1), which were significantly increased in comparison to GSH levels in the controls (p=0.034) and participants with past depression (p=0.013). No differences in GSH levels were evident between controls and participants with past MDD (p=0.846). Groupwise differences in cortisol were also significant (p=0.013, Kruskal Wallis test), and post-hoc comparisons revealed elevated cortisol levels in participants with current (p=0.002) and past MDD (p=0.006) relative to controls, but no significant differences in cortisol levels between the two MDD groups (p=0.893). Although the groups differed in age (p=0.024), no significant correlation was observed between GSH levels and age (rho=-0.141, p=0.267). The correlations with symptom scores revealed a significant negative correlation with PANAS positive score (rho=-0.346, p=0.005), and a trend towards a positive correlation with the perceived stress scale (rho=0.24, p=0.059). No significant correlations were observed between GSH and cortisol (rho=-0.086, p=0.497), or between GSH and depression severity (MADRS: rho=0.144, p=0.261, HAMD: rho=0.158, p=0.215, BDI: rho=0.166, p=0.194).Discussion
Previous studies have shown both increases and decreases of glutathione in depression. Here we observed elevated prefrontal GSH in participants with current but not remitted depression, which was associated with a decrease in positive mood, as well as a trend towards an increase in perceived stress. Cortisol levels were increased in patients with both current and remitted depression, although no association between GSH and cortisol was observed. In light of the modest sample size, particularly for participants with current depression, this study should be replicated in a larger sample. However, the link between elevated glutathione and lower positive affect is supported by previous meta-analyses showing increased glutathione in bipolar disorder11 and provides further evidence for altered glutathione in mood disorders.Acknowledgements
No acknowledgement found.References
- Lim GY, Tam WW, Lu Y. et al. Sci Rep 8, 2861 (2018). https://doi.org/10.1038/s41598-018-21243-x
- Shorey S, Ng ED, Wong CHJ. British Journal of Clinical Psychology 61(2):287-305 (2022)
- Kennedy S. Clin Neurosci. 10(3): 271–277 (2008).
- O’Kean VO, Frodl T, Dinan TG. Psychoneuroendocrinology 37(10):1589-99 (2012).
- Köhler CA, Freitas TH, Maes M, et al. Acta Psychiatrica Scandinavica 135(5):373-387 (2017)
- Luykx JJ, Laban KG, van den Heuvel MP, et al. Neurosci & Biobehav Rev 36(1):198-205 (2012)
- Ritter C, Buchmann A, Müller ST, et al. JAMA Psychiatry 2022 :doi:10.1001/jamapsychiatry.2022.3384.
- Rae CD, Williams SR. Analytical Biochemistry 529:127-143 (2017).
- Bhatt S, Nagappa AN, Patil CR. Drug Discovery Today 25(7):1270-1276 (2020).
- Lapidus KAB, Gabbay V, Mao X, et al. Neurosci Lett 569:74-79 (2014).
- Das TK, Javadzadeh A, Dey A. Prog Neuropsychopharmacol Biol Psychiatry;91:94-102 (2019)
Figures
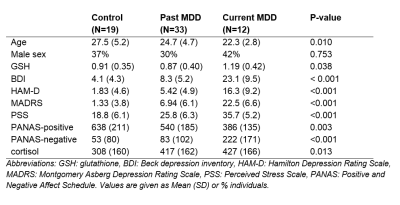
Table 1: Participant demographics, Glutathione (GSH) and cortisol levels, and
symptom severity scores stratified by group.
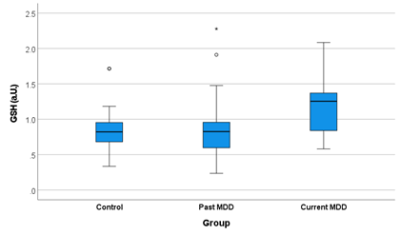
Figure 1. Boxplot depicting the median and interquartile range of the
water-scaled GSH levels in each group, after removal of two outliers (one
control and one participant with past depression). GSH levels were significantly
elevated in the patients with current depression in comparison to those with
past depression and controls (p=0.038, Kruskal Wallis test).
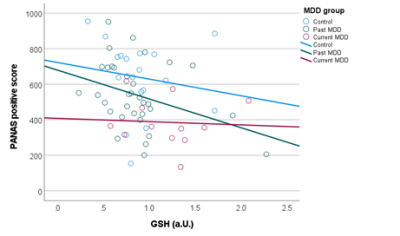
Figure 2. Elevated prefrontal GSH levels were associated with reduced PANAS
positive scores, reflecting a loss of positive mood in participants with higher
GSH concentrations.
DOI: https://doi.org/10.58530/2023/4938