4934
Glymphatic system dysfunction in patients with major depressive disorder1Medical Imaging Department, Nanfang Hospital, Guangzhou, China, 2MR Research, GE Healthcare, Beijing, China, Beijing, China, 3Department of Radiology, Zhujiang Hospital of Southern Medical University, Guangzhou, China, 4Department of Radiology, Zengcheng Branch of Nanfang Hospital, Guangzhou, China
Synopsis
Keywords: Psychiatric Disorders, Brain, Major depressive disorder
Major depressive disorder (MDD) is a severe mental sickness. Yet, its neural mechanisms remain unclear. The present study aimed to analyze the different degrees of glymphatic system activity between patients with MDD and healthy controls using diffusion tension imaging (DTI) along the perivascular space (DTI-ALPS). The results showed that the patients with MDD had glymphatic system dysfunction, which increases anxiety. Thus, the ALPS index is expected to be a biomarker for monitoring changes in glymphatic system function in MDD patients.Introduction
Major depressive disorder, or clinical depression, is a severe mental sickness with a high morbidity rate and relapse rate that can significantly affect individual health. Although its clinical symptoms are well understood, the precise neural mechanisms underlying the development of depression remain elusive.Emerging evidence suggests that blood-brain barrier (BBB) breakdown linked to the glymphatic system may contribute to MDD symptoms1,2. Diffusion tensor imaging has been recently utilized to assess glymphatic system function in several studies. For example, DTI along the perivascular space (DTI-ALPS), which was first proposed by Taoka et al. 3, has shown to be a sensitive approach for evaluating glymphatic system function in patients with neurological diseases3-5; yet, no studies have investigated glymphatic system function in MDD using the ALPS method.
In this study, we evaluated the motion of water molecules in the direction of the perivascular space by measuring diffusivity. The aim of the study was to analyze the different degrees of glymphatic system activity between patients with MDD and healthy controls using the DTI-ALPS calculation method.
Methods
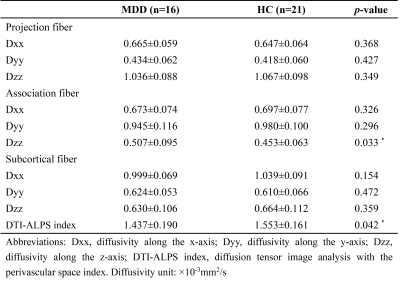
This study included 16 right-handed patients with MDD (2 males and 14 females, aged 18-34 years) and 21 right-handed healthy controls (8 males and 13 females, aged 21-29 years). All subjects were evaluated based on three scales, including 24-items Hamilton Depression Rating Scale (HAMD), 14-items Hamilton Anxiety Rating Scale (HAMA), and Beck Scale for Suicide Ideation (BSS).All patients underwent the same brain MRI protocol on a 3.0T MRI scanner (Signa Architect 3.0T, GE, USA) with the 48-channel head coil. The DTI parameters were following: FOV = 224mm×224mm, TR/TE = 7000/84ms, slice thickness = 2mm, imaging matrix =112×112, acquisition voxel = 2mm×2mm, acquisition layers =62, and diffusion sensitivity factor (b value) = 1000s/mm2. Fractional anisotropy (FA) maps and diffusivity maps of the three directions (x-, y-, and z-axes) were calculated using FSL (www.fmrib.ox.ac.uk/fsl).
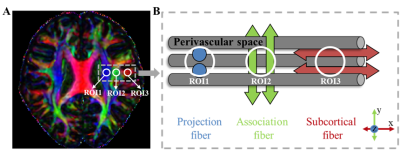
Calculation of ALPS‑index was performed as follows (Fig. 1): on the DTI color-coded FA map in the slices of the lateral ventricle body, three spherical regions of interest (ROI) (diameter = 6mm) were placed in the area with projection fibers, association fibers, and subcortical fibers to measure diffusivities along the three directions (Dxproj, Dyproj, Dzproj, Dxassoc, Dyassoc, Dzassoc, Dxsubc, Dysubc, Dzsubc) using ITK-snap (http://www.itksnap.org/). In this study, we obtained measurements only in the left hemisphere, as all subjects were right-handed3. ALPS-index was calculated using the following formula3: ALPS-index = mean (Dxproj, Dxassoc) / mean (Dyproj, Dzassoc).
The demographic and clinical characteristics were compared using an independent sample Mann-Whitney U test or Fisher's exact test. Comparisons of the diffusivities and ALPS indexes of the groups were performed using an independent sample t-test. Correlation analysis was performed using Pearson’s correlation coefficient. All statistical analyses were performed using ibm spss Statistics 26 (https://www.ibm.com/).
Results
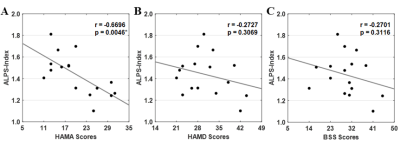
There was no significant difference in age and sex between patients and healthy controls (all p>0.05, Table 1). Group-difference in diffusivities and ALPS index were showed in Table 2. The mean ALPS index was significantly lower in patients with MDD compared to that of healthy controls (1.437 vs. 1.553, p = 0.042). In addition, patients with MDD had higher diffusivity along the z-axis of the association fiber than the healthy controls (0.507×10-3 vs. 0.453×10-3, p = 0.033). Also, the ALPS index was negatively correlated with HAMA scores in patients with MDD (r = -0.6696, p = 0.0046, Fig. 2), but not with other clinical characteristics, including HAMD scores and BSS scores (r = −0.2727, p = 0.3069; r = −0.2701, p = 0.3116, respectively).Discussion
In this study, we found that the mean ALPS index for patients with MDD was significantly lower than that for healthy controls, which confirmed that the patients with MDD have glymphatic system dysfunction. In a previous study, Ranti et al. explored glymphatic system function in MDD using the Virchow Robin space (VRS, i.e., perivascular compartments surrounding small cerebral blood vessels) count1. They found that cerebrospinal fluid-brain interstitial fluid exchange occurs via the brain-wide network of PVSs, which constitute the glymphatic system. In this study, we found that the ALPS index was negatively correlated with HAMA scores in patients with MDD, which suggested that the glymphatic system function of these patients gradually declines while the level of anxiety symptoms increases. MDD commonly co-occurs with clinically significant levels of anxiety6,7. A previous study showed that the prevalence of anxiety symptoms in first-episode MDD patients was 79.2%8. In contrast, the ALPS index of patients with MDD was not correlated with HAMD scores and BSS scores. Yet, further studies with large sample sizes are needed to confirm these findings.Conclusion
This study shows that patients with MDD have glymphatic system dysfunction, which is significantly correlated with the level of anxiety symptoms. Thus, the ALPS index might be used as a potential biomarker for monitoring glymphatic system function in patients with MDD.Acknowledgements
This study was supported by the National Natural Science Foundation of China grant 82172012.References
1. Ranti DL, Warburton AJ, Rutland JW et al. Perivascular spaces as a marker of psychological trauma in depression: A 7‐Tesla MRI study. Brain Behav. 2022;12(7):32598
2. Iliff JJ, Wang M, Liao Y et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111
3. Taoka T, Masutani Y, Kawai H et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172-178
4. Zhang W, Zhou Y, Wang J et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257
5. Lee H, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in patients with juvenile myoclonic epilepsy. J Neurol. 2022;269(4):2133-2139
6. Braund TA, Palmer DM, Williams LM, Harris AWF. Dimensions of anxiety in Major depressive disorder and their use in predicting antidepressant treatment outcome: an iSPOT-D report. Psychol Med. 2020;50(6):1032-1042
7. Gaspersz R, Nawijn L, Lamers F, Penninx BWJH. Patients with anxious depression. Curr Opin Psychiatr. 2018;31(1):17-25
8. Yang W, Zhang G, Jia Q et al. Prevalence and clinical profiles of comorbid anxiety in first episode and drug naïve patients with major depressive disorder. J Affect Disorders. 2019;257:200-206
Figures