4930
Alterations of the structural covariance network in the thalamic subnuclei of non-comorbid treatment-naïve patients with major depressive disorder1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Department of Psychiatry, West China Hospital of Sichuan University, Chengdu, China
Synopsis
Keywords: Psychiatric Disorders, Gray Matter
The thalamus is one of the key structures involved in the pathophysiology of major depressive disorder (MDD). Most previous neuroimaging studies on MDD measured the volume of thalamus as a whole, however, the thalamus consists of multiple subnucleus with distinct function and connectivity. Recent advances in structural MRI segmentation techniques have enabled the measurement of the volumes of thalamic subnucleus with a robust, automatic approach using a Bayesian inference-based atlas-building algorithm. Using this algorithm, we aimed to investigate the alternations in volume and structural covariance networks in thalamic subnucleus in a relatively large sample of non-comorbid treatment-naïve patients with MDD.Background
The thalamus is one of the key structures in the limbic-cortical-striatial-pallidal-thalamic (LCSPT) circuit and the LCSPT circuit dysfunction has been found to be centrally implicated in major depressive disorder (MDD)1-3. Most prior structural imaging studies of MDD obtained measurements from the whole thalamus1, 4, 5, however, the thalamus consists of several histologically and functionally distinct subnucleus 6, 7. Further, the available literature is not consistent, with reports of both total thalamus volume decrease and increase reported in case-control studies1, 8. The discrepancies could be potentially as a result of effects of treatment, illness progression and comorbidities that can variably impact brain morphometry and confound measurement of illness-related features. Whole-brain structural covariance abnormalities were reported previously in patients with MDD9, 10. However, no study to date has focused on the structural covariance network within the thalamus. Hence, in the current study, we recruited a relatively large sample of non-comorbid treatment-naïve patients with MDD to investigate the alternations in volume and structural covariance networks in thalamic subnucleus.Materials and methods
We collected high-resolution 3D T1-weighted images from 81 untreated patients with 123 non-comorbid treatment-naïve patients with MDD and 81 age-, sex-, handedness matched healthy controls (HCs) (Table1). The 17-item Hamilton Depression Scale (HAMD-17) and 14-item Hamilton Anxiety Scale (HAMA-14) were used to evaluate the severity of depression and anxiety in patients with MDD, respectively. All MRI scans were performed using a Siemens 3.0 T MRI scanner. High-resolution, T1-weighted images were obtained with a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with the following acquisition parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.5 ms, inversion time (TI) = 900 ms, flip angle = 9°, matrix = 256×256, field of view (FOV) = 256×256 mm2, number of slices = 176, and slice thickness =1.0 mm.The structural data was automatically segmented using FreeSurfer software (V 6.0). Thalamic subnucleus segmentation was performed using a special purpose module in FreeSurfer software which employs a tetrahedral mesh-based probabilistic atlas built from manually delineated amygdala in in-vivo and ex-vivo data 6. We obtained the volumes of the left and right thalamus and 50 bilateral subnucleus (Fig 1). All segmentations were visually inspected according to the ENIGMA control protocol (http://enigma.ini.usc.edu/). No MRI measurements for study participants as described above showed signs of software failure in thalamic subnucleus measurements. We conducted multivariate analysis of covariance (MANCOVA) to test for groups differences in the overall thalamic volume and thalamic subnucleus volumes, with age, sex, education level and intracranial volume (ICV) as covariates. Partial eta squared (η2) was used to evaluate effect size (0.01 indicates a small effect size, 0.06 indicates a medium effect size and 0.14 indicates a large effect size). Partial correlation analyses were performed to identify clinical associations of thalamic measures that showed significant group differences with age of onset, illness duration, HAMD and HAMA scores in the patients with MDD, controlling for age, sex, education level and ICV. We also performed an analysis of the structural covariance network in the thalamus using graph theory and the BRAPH program (BRAPH; version 1.00; http://braph.org/)10.The global and local thalamic network measures (characteristic path length, global efficiency, local efficiency, clustering coefficient, small-worldness index) were compared by testing the statistical significance of the differences using nonparametric permutation tests with 1000 permutations. A false discovery rate (FDR) correction was applied to correct for multiple hypothesis testing issues in between-group comparison and correlation analyses.
Results
The whole thalamic volumes of the left and right hemispheres were no significantly different between the MDD and HC groups (Table 2). Follow-up subnucleus analyses showed that smaller volume in the left medial geniculate nucleus (MGN) and left pulvinar lateral (PUL) nucleus, while large volume in the right central lateral (CL) nucleus in the patients with MDD compared to HCs (FDR corrected p<0.05,Table 2, Fig. 2). No significant correlations were observed between abnormal thalamic measures and clinical characteristics of MDD.The global network measures of characteristic path length, global efficiency, local efficiency and clustering coefficient in MDD were lower than those in HCs (FDR corrected p<0.05, Table 3).
Discussion and Conclusion
In this study, we investigated the thalamus at the subnucleus level and alterations in the structural covariance network in the thalamus in a relatively large sample of non-comorbid treatment-naïve patients with MDD. There were two primary findings that emerged from this study. First, we observed that the volume of left MGN and PUL was significantly smaller, while the volume of right CL was significantly larger in the patients with MDD relative to HCs. Second, the global network measures of characteristic path length, global efficiency, local efficiency of and clustering coefficient were lower than those in HCs. These findings demonstrated MDD may lead to disruption in posterior and intralaminar thalamus which are involved in auditory fear conditioning, visual attention and emotion regulation. Moreover, the structural covariance network of thalamic subnucleus of patients with MDD was reorganized, and the transmission efficiency was weakened. Our study may provide a new perspective for the pathogenesis of MDD.Acknowledgements
This study is supported by grants from 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21041) and Clinical and Translational Research Fund of Chinese Academy of Medical Sciences (2021-I2M-C&T-B-097).
References
1. Nugent, A.C., et al., Reduced thalamic volumes in major depressive disorder. Psychiatry Res, 2013. 213(3): p. 179-85.
2. Taber, K.H., et al., The limbic thalamus. J Neuropsychiatry Clin Neurosci, 2004. 16(2): p. 127-32.
3. Drevets, W.C., J.L. Price, and M.L. Furey, Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct, 2008. 213(1-2): p. 93-118.
4. Bora, E., et al., Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med, 2012. 42(4): p. 671-81.
5. Lu, Y., et al., The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. Neuroimage Clin, 2016. 11: p. 658-666.
6. Iglesias, J.E., et al., A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage, 2018. 183: p. 314-326.
7. Behrens, T.E., et al., Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci, 2003. 6(7): p. 750-7.
8. Zhang, X., et al., Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. J Affect Disord, 2012. 136(3): p. 443-52.
9. Watanabe, K., et al., Whole-brain structural covariance network abnormality in first-episode and drug-naïve major depressive disorder. Psychiatry Res Neuroimaging, 2020. 300: p. 111083.
10. Xiong, G., et al., Potential structural trait markers of depression in the form of alterations in the structures of subcortical nuclei and structural covariance network properties. Neuroimage Clin, 2021. 32: p. 102871.
Figures
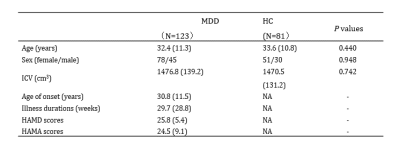
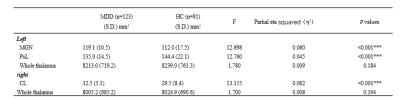
Table 2. Thalamic subnucleus volumes (mm3) in MDD patients and HCs. MDD, major depressive disorder; HCs, health controls; MGN, medial geniculate nucleus; PUL, pulvinar lateral; CL, central lateral. *** indicates p<0.001.
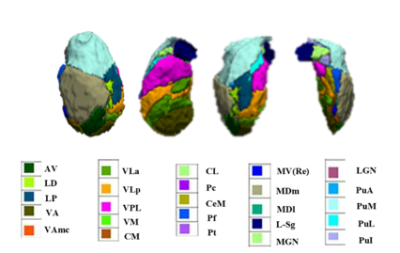
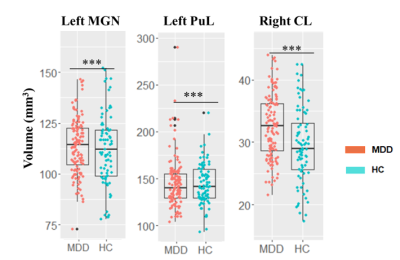
Figure 2. Barplots of volumes of thalamic subnucleus in patients with MDD compared with HCs, adjusted for age, sex, education level and ICV. MDD, major depressive disorder; HCs, health controls; MGN, medial geniculate nucleus; PUL, pulvinar lateral; CL, central lateral. *** indicates p<0.001.