4896
Monte Carlo simulations of transverse relaxation for assessing physico-chemical properties of novel superparamagnetic iron oxide particles1Clinic of Radiology, University of Münster, Münster, Germany, 2Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas, Universidad Nacional de La Plata - CONICET, La Plata, Argentina, 3Institut für Anorganische und Analytische Chemie, University of Münster, Münster, Germany, 4CeNTech, CiMIC, SoN, University of Münster, Münster, Germany
Synopsis
Keywords: Relaxometry, Contrast Agent, Simulations
Physico-chemical properties of superparamagnetic iron oxide nanoparticles (IONP) strongly affect their impact on transverse relaxation and their efficiency as MR contrast agents. Monte Carlo simulations were developed to model relaxation in silico for two novel IONPs with distinct physico-chemical properties. The IONPs comprised an identical magnetite core but differed in preparation strategy, defining their coating and size. Simulations were compared to in vitro relaxometry measurements and reproduced experimentally measured relaxation times. Using lognormally distributed particle radii in the simulations was required to obtain correct R2* values. Together, simulations and measurements unveiled additional particle aggregation in the MR samples.
Introduction
Superparamagnetic iron oxide nanoparticles (IONP) are versatile MRI contrast agents for applications in cell tracking, blood volume measurement or assessment of barrier function. Relaxation time mapping enables quantification of local effects of IONPs on MR relaxation. However, concentration or spatial distribution of IONPs cannot be directly derived from such measurements since relaxation times depend on their exact physico-chemical properties1. Here, we implemented a simulation pipeline to characterize the relation between relaxation times and physico-chemical properties for two IONPs.Methods
Monte Carlo simulations were implemented to predict the change of transverse relaxation times T2/T2* in the presence of IONP-based contrast agents with distinct physico-chemical properties2,3 (Fig. 1). Inside a cubic voxel (0.000001–0.001 mm3) IONPs were modeled as randomly distributed impermeable spheres, giving rise to local dipole fields. Diffusion of 5,000 to 10,000 water molecules was modeled as random walk for 100 ms. The phase of each spin was accrued over a given number of diffusion steps (100,000–10,000,000) through the local dipole fields. The MR signal at each diffusion step was obtained by summing over all simulated spins and T2* was calculated with an exponential fit of the signal. For T2 simulations, a multi-spin-echo-sequence (echo spacing: 6.5 ms, 15 echoes) was implemented and phases of all spins were inverted after each refocusing pulse.Simulations were parameterized to reproduce two nanohybrid particles, which both comprised an identical superparamagnetic magnetite core of ~16 nm diameter with a saturation magnetization of 62 Am2/kg. The two IONPs differed in preparation strategies, one is based on encapsulation of the IONP with an amphiphilic comb polymer poly(maleic anhydride alt-1-octadecene) (PMAO) and the other involves a ligand-exchange procedure at the IONP using tetramethyl ammonium hydroxide (TMAOH). The hydrodynamic radii of both particles were lognormally distributed, as measured by dynamic light scattering (DLS) (Fig. 2). Average radii of PMAO- and TMAOH-particles were 76 nm and 130 nm, respectively.
For MRI, the particles were prepared in agarose phantoms in iron concentrations of 90, 180 and 400 µmol/L. Measurements were performed on a 9.4 T animal scanner (Bruker BioSpec 94/20). For T2 measurements, a spin-echo-sequence with variable echo times (TE, min: 6.5 ms, echo spacing: 6.5 ms, 16 echoes, TR: 2000 ms) and for T2* measurements ultra-short-echo-sequences (TE, min: 0.45 ms, TE, max: 40 ms, TR: 120 ms, flip angle: 20°) were used.
Simulation of the MR measurements was performed for concentrations of 90, 180 and 400 µmol/L Fe, for constant radii of 76 nm and 130 nm, and for lognormally distributed hydrodynamic radii. To account for potential particle aggregation, simulations were performed with different hydrodynamic radii from 30 to 500 nm. Each simulation took between 30 s to 140 min on a high-performance-computing cluster and was repeated three times.
Results
MRI measurements showed linearly increasing transverse relaxation rates R2/R2*, with higher concentration for both IONPs (Fig. 2, 3). Relaxivities were r2 = 422.3 $$$\pm$$$ 4.3 Lmmol-1s-1, r2* = 634 $$$\pm$$$ 16 Lmmol-1s-1 for PMAO-particles and r2 = 74 $$$\pm$$$ 19 Lmmol-1s-1, r2* = 862.0 $$$\pm$$$ 9.8 Lmmol-1s-1 for TMAOH-particles. Relatively large differences between R2 and R2* were observed for TMAOH-particles.Simulations of the MR experiments reproduced the linear correlation between the concentration and R2/R2* for both IONPs (Fig. 3). Simulations with constant radii reproduced R2/R2* values for the PMAO-particles well but differed substantially for higher concentrations of the TMAOH-particles. Using distributed hydrodynamic radii, had minor effects on R2, but increased R2*, which for the TMAOH-particles perfectly matched the measured data.
Simulations of different hydrodynamic radii showed, that R2* was largely independent of the radius, whereas R2 decreased with increasing radius (Fig. 4). While the simulation reproduced measured R2* for both particles, measured R2 values for the TMAOH-particles were lower, suggesting larger particle radii (distributed around 300 nm) than observed with DLS. High resolution MRI of a TMAOH phantom sample indeed suggested substantial particle aggregation (Fig. 5).
Discussion
The implemented simulations were able to reproduce experimentally measured R2/R2*. Relaxation rates increased for higher IONP concentrations and for larger radii (TMAOH, but not PMAO) a pronounced difference between R2 and R2* was reproduced.Using lognormally distributed radii hardly affected R2 values. However, for R2*, larger values were obtained that matched the experimental data for the TMAOH-particles. The remaining differences in R2 for the TMAOH-particles can be explained by particle aggregation with radii distributed around 300 nm (Fig. 4, 5).
Both, measured and simulated differences in R2 and R2* for the IONPs agree with relaxation theory4,5. Small IONPs are described by the motional averaging regime (MAR), where R2 and R2* are comparable and increase with increasing radius. Larger IONPs fall in the static dephasing regime (SDR), where R2* is independent of the radius, and the echo-limited regime (ELR), where R2 decreases with increasing radius. PMAO-particle radii are between the MAR and SDR, while TMAOH-particle radii fall in the SDR and ELR.
Conclusion
Our simulations are suitable to quantify the influence of IONP-based contrast agents on transverse relaxation times. Here, the combination of measurements and simulations was able to unveil particle aggregation from known concentrations. In future cell tracking studies with known intracellular iron distribution, our simulations may enable exact quantification of infiltrated cells in a given tissue region.Acknowledgements
No acknowledgement found.References
- Masthoff M, Buchholz R, Beuker A, et al. Introducing Specificity to Iron Oxide Nanoparticle Imaging by Combining 57Fe-Based MRI and Mass Spectrometry. Nano Lett. 2019;19(11):7908-7917.
- Vuong QL, Gillis P, Gossuin Y. Monte Carlo simulation and theory of proton NMR transverse relaxation induced by aggregation of magnetic particles used as MRI contrast agents. Journal of Magnetic Resonance. 2011;212(1):139-148.
- Matsumoto Y, Jasanoff A. T2 relaxation induced by clusters of superparamagnetic nanoparticles: Monte Carlo simulations. Magnetic Resonance Imaging. 2008;26(7):994-998.
- Vuong QL, Gillis P, Roch A, Gossuin Y. Magnetic resonance relaxation induced by superparamagnetic particles used as contrast agents in magnetic resonance imaging: a theoretical review. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(6).
- Brooks RA. T(2)-shortening by strongly magnetized spheres: a chemical exchange model. Magn Reson Med. 2002;47(2):388-391.
Figures
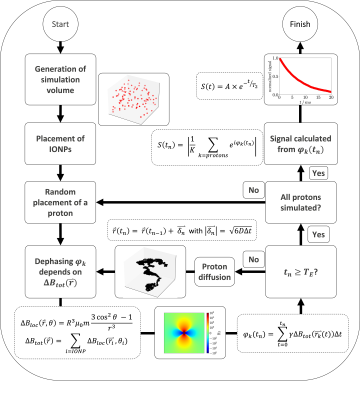
Figure 1: Flowchart of the Monte Carlo
simulation, to simulate the influence of superparamagnetic iron oxide
nanoparticle (IONP) based contrast agents on the transverse relaxation
time of water. $$$\Delta B_{loc}(\vec{r})$$$ is the local
change in total magnetic field caused by one IONP, $$$\Delta
B_{tot}(\vec{r})$$$ the total change in magnetic field, $$$\varphi_k$$$ the phase of proton $$$k$$$, $$$t_n$$$ the current time step $$$n$$$, $$$T_E$$$ the echo time and $$$S(t_n)$$$ the resulting MR signal.
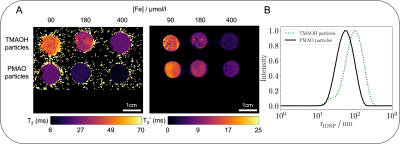
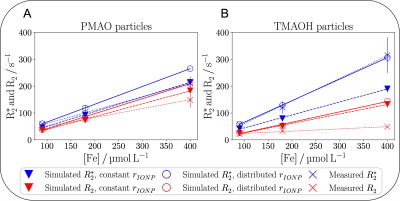
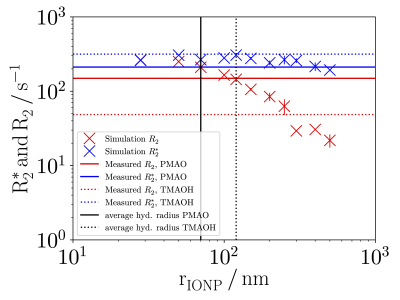
Figure 4: Simulation results of relaxation rates for different lognormally distributed hydrodynamic radii rIONP. Mean simulated R2 (blue X) and R2*
(red X) with vertical lines indicating standard deviation from three
simulation runs. Black vertical lines indicate average hydrodynamic
radii of PMAO- (solid lines) and TMAOH-particles (dotted lines) as
obtained by DLS. Horizontal lines indicate experimental mean R2 (blue) and R2* (red) of PMAO- (solid lines) and TMAOH-particles (dotted lines) as obtained by MRI.
