4890
Deep Learning-based Flexible Echo Time Dual-Echo Water-Fat Separation
Yan Wu1, Zhitao Li1, Marcus Alley1, Zhifei Wen2, Zheng Zhong1, Fan Zhang3, John Pauly1, and Shreyas Vasanawala1
1Radiology, Stanford University, Stanford, CA, United States, 2Hoag Hospital, Newport Beach, CA, United States, 3Radiology, Stanford Children's Hospital, Stanford, CA, United States
1Radiology, Stanford University, Stanford, CA, United States, 2Hoag Hospital, Newport Beach, CA, United States, 3Radiology, Stanford Children's Hospital, Stanford, CA, United States
Synopsis
Keywords: Fat, Fat, deep learning, dual-echo water-fat separation, flexible echo time
We designed a deep learning-based dual-echo water-fat separation method with capability to support flexible echo times. A densely connected hierarchical network was employed, where input included dual-echo images and echo times, and ground truth images were produced using the projected power method. The model was trained and tested using 78 contrast enhanced image sets acquired with optimal echo times, and further validated on 15 non-contrast enhanced image sets obtained with different imaging parameter values. The proposed water-fat separation method has demonstrated high accuracy when dual-echo images were acquired with optimal or non-optimal echo times.INTRODUCTION
In dual-echo imaging, the prescription of a high in-plane resolution or a low receiver bandwidth may force the echo times (TEs) to deviate from their minimal optimal values. To avoid incomplete water-fat separation, the echo times were usually extended to next optimal values, resulting in an elongated scan. To facilitate more efficient data acquisition, we propose a deep learning-based dual-echo water-fat separation approach that supports flexible echo times with a better tolerance of non-optimal TE combinations than the traditional algorithms.METHODS
Deep Learning Model for Dual-Echo Water-Fat SeparationIn this study, we investigated a deep learning-based flexible echo time water-fat separation approach. For the proposed task, a deep neural network was employed, where the input included complex dual-echo images as well as the corresponding echo times, and the ground truth water/fat images were produced from the dual-echo images using the conventional projected power method (a robust binary quadratic optimization approach [1]), as illustrated in Figure 1.
A unique design of the proposed deep learning method was to include imaging parameters as additional network input. For every slice, not only were dual-echo images used as input, but also images that provided in-phase and opposed-phase echo times at every pixel.
The network was a densely connected hierarchical convolutional neural network with multiple outputs, which simultaneously derived water and fat images from dual-echo images. It was similar to T-Net [2] except having several 1×1 kernels at the last layer for the production of multiple outputs.
Data Acquisition
With IRB approval and informed patient consent, contrast enhanced dual-echo images of the extremities (knees, ankles, arms, hands) were acquired using a 3D SPGR sequence. Based upon prescribed image resolution and system gradient strength, two cluster of opposed-phase TEs values were used (1.25 - 1.31ms or 3.35ms). Meanwhile, an echo time of 2.23ms was used to acquire in-phase images. Other imaging parameters were as follows: bandwidth = 192 kHz, FOV = 32 36cm, matrix size = 512 512, number of slices = 292 – 440, slice thickness = 1mm, flip angle = 15, scan time = 2 min 48 sec – 6 min 10 sec for a 3D image volume.
To investigate the model’s capability to support flexible flexible echo times, we acquired a series of non-contrast enhanced dual-echo image sets from each of the volunteers using different imaging parameter values.
Model Training and Testing
In contrast enhanced images acquired using optimal echo times from 78 patients (21238 two dimensional images), data from 60 subjects were used for training, and the rest were used for testing. In addition, 15 series of non-contrast enhanced images obtained with different echo times from 3 healthy volunteers (5880 two dimensional images) were used to investigate the model’s capability to support flexible echo times.
The L1 loss was employed as the loss function. The network parameters were updated using the Adam algorithm with alpha of 0.001, beta1 of 0.89, beta2 of 0.89, and e of 10^-8.
RESULTS
A deep learning-based dual-echo water-fat separation model was trained and tested. Using the proposed deep learning method, the data processing time required for a 2D image was substantially reduced to 0.13 seconds (from 1.5 seconds using the projected power). High fidelity was achieved with an averaged correlation coefficient of 0.99, error of 0.03, and SSIM of 0.95.The proposed method provides accurate water-fat separation. An example of contrast enhanced hand images is demonstrated in Figure 3. In general, water-fat separation in the hand is challenging due to relatively severe B0 inhomogeneities. In this contrast enhanced study, the predicted image was improved over the reference image, particularly in the region of fingertips where B0 inhomogeneities are more apparent. This confirms the advantage of deep learning in B0 estimation [3,4].
Using the established model, even if the imaging parameters of test images were different from those adopted in training sets, the predicted images were accurate. In Figure 4, water images derived from dual-echo images acquired with different imaging parameters (an acceleration factor of 2, bandwidth of 83.3 kHz, flip angle of 25°, or phase encoding of 224) were similar to the reference images. When bad shimming was intentionally imposed (to generate highly inhomogeneous B0 field), water/fat swaps that occurred in reference images were corrected in the predicted images.
When echo times of dual-echo images deviated from the optimal values, the deep learning method still worked well. Figure 5 presents a case with different echo times applied. With the optimal echo times (TEs=1.2/2.3ms), water/fat images derived using deep learning and conventional methods were very similar. With non-optimal echo times (TEs=1.7/3.0ms), the conventional projected power method failed in water-fat separation, whereas the deep learning method was significantly improved with only minor water/fat swaps appearing.
DISCUSSION
The proposed method maintained high accuracy with the use of flexible imaging parameters. Particularly interesting is the support to non-optimal echo times, which will facilitate more efficient acquisition of high-resolution images, which was made possible with the echo times incorporated as additional network input.CONCLUSIONS
A deep learning-based dual-echo water-fat separation method is developed, which has a capability to support flexible echo times and facilitate more efficient acquisition of high-resolution images.Acknowledgements
The research was supported by National Institute of Health: NIH R01EB009690, NIH R01 EB026136, NIH R01DK117354 and GE Healthcare.References
1. Zhang, Tao, et al. "Resolving phase ambiguity in dual‐echo dixon imaging using a projected power method." Magnetic resonance in medicine 77.5 (2017): 2066-2076. 2. Wu Y, et al. Deep Learning-Based Water-Fat Separation from Dual-Echo Chemical Shift-Encoded Imaging. Bioengineering. 2022 Oct 19;9(10):579. 3. Y. Wu, et al., "Incorporating prior knowledge via volumetric deep residual network to optimize the reconstruction of sparsely sampled MRI," Magnetic resonance imaging, 2019. 4. Wu, Yan, et al. "Quantitative Parametric Mapping of Tissues Properties from Standard Magnetic Resonance Imaging Enabled by Deep Learning." arXiv preprint arXiv:2108.04912 (2021).Figures
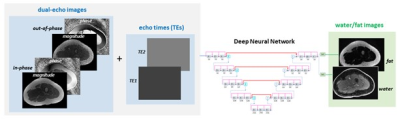
Deep learning-based water-fat separation from
dual-echo images. A deep neural network was employed to provide end-to-end
mapping from dual-echo images and related echo times to the corresponding
water/fat images.
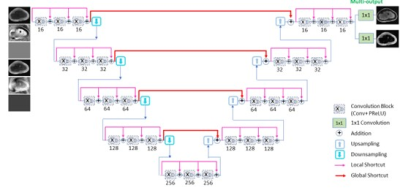
A densely connected hierarchical multi-output deep
convolutional neural network for water-fat-separation.
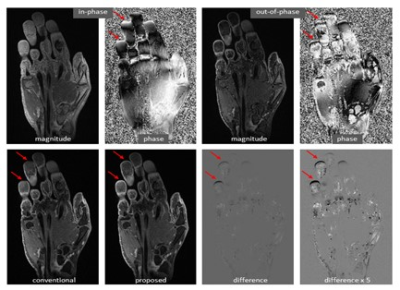
Improvement of a predicted hand image over the reference image, particularly
in the region of fingertips where B0 inhomogeneities are more apparent.
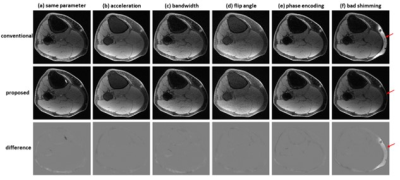
Water-fat separation from dual-echo images acquired
using different parameters. (a) the same imaging parameters as those adopted in
contrast enhanced studies, (b) an acceleration factor of 2, (c) bandwidth of
83.3 kHz, (d) flip angle of 25°, (e) phase encoding of 224. In these cases, the
predicted images had high fidelity to the reference images. (f) When bad
shimming was intentionally imposed, water/fat swaps that occurred in the
reference image were corrected in the predicted image.
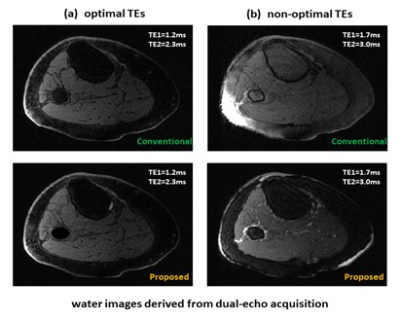
Water images derived from non-contrast enhanced dual-echo
images acquired with optimal or non-optimal echo times. (a) with the employment
of the optimal echo times of 1.2/2.3ms, water images derived using the deep
learning model or conventional method are very similar. (b) with the employment
of non-optimal echo times of 1.7/3.0ms, the conventional projected power method
failed, whereas the deep learning method was significantly improved with only
minor water/fat swaps appearing.
DOI: https://doi.org/10.58530/2023/4890