4888
The magnetic resonance characteristics of SPIONs for imaging at ultralow field (6.5 mT)1MGH/A. A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 2The University of Sydney, Sydney, Australia, 3Imagion Biosystems, Ltd, San Diego, CA, United States, 4Department of Physics, Harvard University, Cambridge, MA, United States, 5Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Contrast Agent, Modelling
Characterization of the parameters of superparamagnetic iron oxide nanoparticles (SPIONs) can guide synthesis of optimized particles for use in magnetic resonance imaging (MRI). With a goal of application of SPIONs in ultra-low field (ULF) MRI, we investigated the properties of SPIONs, and conducted imaging on a 6.5 mT MRI scanner. Specifically, we measured the NMR relaxivity and susceptibility of different SPIONs sample, both dominated by the core size of SPIONs. We also implemented susceptibility imaging using an bSSFP sequence to obtain positive contrast images of SPION phantoms, and present here a method to optimize the ULF susceptibility MRI images.Introduction
MRI in the millitesla regime promises to enable MRI in outside of the controlled access scanner suite in locations such as the ICU, inpatient ward, and ambulance, and provide mobile and affordable MRI in low resource regions1,2,3. SPIONs can effectively enhance the contrast of MRI images4,5, even at ultra-low magnetic fields where typical Gd-based contrast agents don’t provide enough contrast6. Study of the magnetic resonance parameters of SPIONs at ultra-low field can guide the synthesis and modification of SPIONs to enable the design of optimized agents for application in ULF MRI.In this work, we characterized the relaxivity and susceptibility of different kinds of SPIONs produced by Imagion Biosystems and demonstrate positive contrast MRI images of SPION in a phantom via susceptibility MRI. Specifically, the relaxivity was measured on a 6.5 mT MRI scanner and the susceptibility of the SPION sample was determined by combining electromagnetic simulations with MRI experimental results. Susceptibility imaging was implemented using balanced steady-state free precession (bSSFP) sequence to obtain positive contrast MRI images, and we optimized the sequence parameters by MRI simulation and imaging experiments. Our work demonstrates that the relaxivity and susceptibility of SPIONs mainly depend on the core size of SPIONs at 6.5 mT. Means to modify the contrast of MRI images by adjusting the parameters of bSSFP sequence are also shown.
Method
In this work, we measured the relaxivity and susceptibility of SPIONs and then implemented bSSFP-based susceptibility imaging and optimized the acquisition parameters.Solutions of SPION samples were diluted variously into deionized water and agarose, and the longitudinal and transverse recovery time T1 and T2 were measured using a spectroscopic inversion recovery or a Carr-Purcell-Meiboom-Gill (CPMG) sequence, respectively. The longitudinal and transverse relaxivity of these samples is then calculated by formula (1). Where T1,2 is the appropriate recovery time of SPIONs in aqueous solution or agarose sample, T10,20 is the recovery time of solvent (deionized water or agarose), R1,2 denotes the relaxivity, and [CA] denotes the concentration of SPIONs sample.
$$\frac{1}{T_{1,2}}=\frac{1}{T_{10,20}}+R_{1,2}[CA]\quad\quad\quad(1)$$
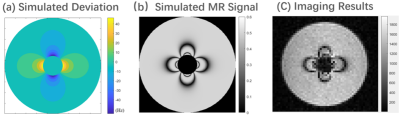
The susceptibility of the SPIONs sample is evaluated by pixel-based fit of the acquired MRI images phantom to the simulated MRI images7. We designed a phantom consisting of a test tube filled with SPION sample under test surrounded by a large cylinder filled with deionized water. A schematic of the phantom is shown in Fig. 1. The unique susceptibility of the SPION sample changes the local magnetic field distribution in the imaging region. The magnetic field deviation map can be analytically calculated and is shown as Fig. 2(a). We used the signal magnitude of the MR images in the region of the deionized water to evaluate the susceptibility of SPION sample. The signal magnitude of MR images obtained with bSSFP sequence was simulated via the Bloch equations, and the simulated MR images is shown as Fig. 2(b). We obtain the susceptibility of SPION sample from analysis of the imaging results, shown in Fig. 2(c).
We also implemented simulations and experiments to understand the relationship between the parameters of bSSFP sequence and the contrast characteristics of MRI images.
Results
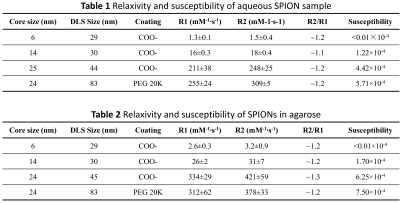
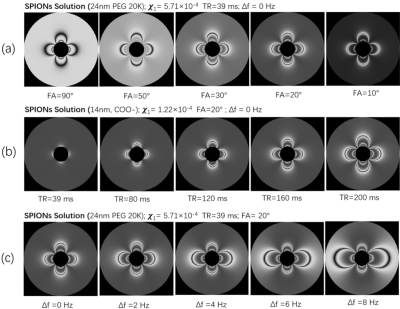
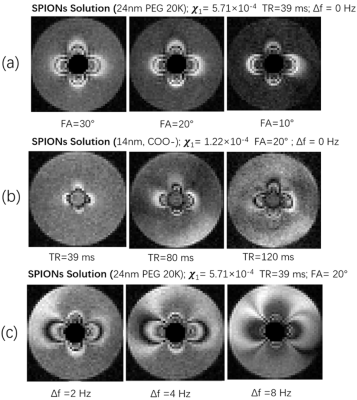
We present the relaxivity and the susceptibility of SPION in aqueous solution and in agarose in table 1 and table 2, respectively. The susceptibility of SPIONs sample was measured with the concentration of SPIONs of 100 ug/mL Fe2O3.Variation of the flip angle (FA), repetition time (TR), and off-resonance frequency (Δf) of the bSSFP sequence was implemented the MRI simulation. The results are shown Fig. 3. These simulations were validated via MRI experiments, acquired with the same parameters as the simulation are shown as Fig. 4. The matrix size was set to 62 × 40 × 5, resolution=2 mm × 2 mm ×10 mm, time of echo (TE)=20 ms, and number of averages=40. The total acquisition time is around 6 minutes.
Discussion
Our study demonstrates that the relaxivity and susceptibility of the SPION samples under study was mainly determined by the core size of SPION. The coating specifics also influences the relaxivity and susceptibility but to a much smaller extent.For bSSFP-based susceptibility imaging, we show that the contrast of MR images can be changed by adjusting the flip angle of pulse sequence where when operating on resonance, large flip angles typically provide negative contrast and small flip angle provide a bright contrast. The susceptibility selective effect on imaging can be accumulated by increasing the repetition time of pulse sequence, and off-resonance operation can be used to to select a region of specific susceptibility in the MR images.
Large core-size SPIONs are recommended for operation at ULF since local susceptibility and magnetic resonance parameters are most strongly impacted. Anticipating in vivo use, uptake varies in terms of the core size of SPIONS8, and if a particular case requires use of small core particles, increasing TR can enable ultra-low field susceptibility MRI in vivo.
Conclusion
We have characterized the relaxivity and susceptibility of different SPIONs at ultralow field, and demonstrated they have a significant core size dependency. Adjusting and optimizing the parameters of bSSFP signal can give a bright contrast to the MRI images of SPIONs phantom and modify the bright contrast in the images.Acknowledgements
This work was supported in part by a sponsored research agreement with Imagion Biosystems, Inc. MSR acknowledges the gracious support of the Kiyomi and Ed Baird MGH Research Scholar award.References
[1] M. Sarracanie, C. D. LaPierre, N. Salameh, D. E. J. Waddington, T. Witzel, and M. S. Rosen, “Low-Cost High-Performance MRI,” Sci Rep, vol. 5, no. 1, p. 15177, Oct. 2015.
[2] S. Geethanath and J. T. Vaughan, “Accessible magnetic resonance imaging: A review,” Journal of Magnetic Resonance Imaging, vol. 242, p. 190, Jan. 2019.
[3] J. P. Marques, F. F. J. Simonis, and A. G. Webb, “Low-field MRI: An MR physics perspective,” Journal of Magnetic Resonance Imaging, vol. 58, p. 1182, Jan. 2019.
[4] M. Borges et al., “Dual T 1 /T 2 MRI contrast agent based on hybrid SPION@coordination polymer nanoparticles,” RSC Adv., vol. 5, no. 105, pp. 86779–86783, 2015, doi: 10.1039/C5RA17661A.
[5] Girard OM, Du J, Agemy L, Sugahara KN, Kotamraju VR, Ruoslahti E, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: comparison of T1,T2*, and synergistic T1-T2* contrast mechanisms. Magn Reson Med 2011; 65:1649–60.
[6] D. E. J. Waddington, T. Boele, R. Maschmeyer, Z. Kuncic, and M. S. Rosen, “High-sensitivity in vivo contrast for ultra-low field magnetic resonance imaging using superparamagnetic iron oxide nanoparticles,” Sci. Adv., vol. 6, no. 29, p. eabb0998, Jul. 2020, doi: 10.1126/sciadv.abb0998.
[7] P. Cantillon-Murphy, L. L. Wald, M. Zahn, and E. Adalsteinsson, “Measuring SPIO and Gd contrast agent magnetization using 3 T MRI,” NMR in Biomedicine, vol. 22, no. 8, pp. 891–897, 2009, doi: 10.1002/nbm.1412.
[8] Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11(11):2319-31. doi: 10.1007/s003300100908. PMID: 11702180.
Figures