4887
A MMP-Responsive Nanoplatform with Transformable Magnetic Resonance Property for Quantitative Tumor Bioimaging and Synergetic Therapy1Shanghai General Hospital,School of Medicine ,Shanghai jiaotong University, Shanghai, China, 2Lthink Medical Institute, Guangzhou, China, 3Philips Healthcare, Shanghai, China
Synopsis
Keywords: Molecular Imaging, Multimodal
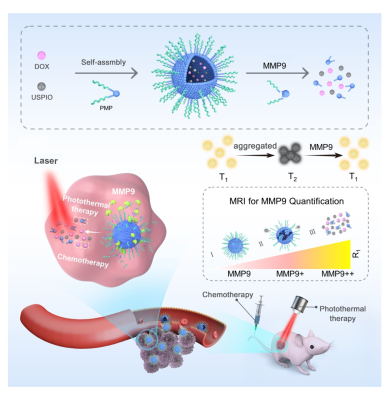
We developed a novel nanoplatform-based MMP9-responsive T2–T1 switching MRI contrast agent, which can be used not only for non-invasive visualization and quantitative analysis of MMP9 activity, but also as a carrier in photothermal sensitization chemotherapy. The nanoplatform acts as a T2 contrast agent in physiological conditions ,then transform into a T1 contrast agent by MMP9 stimulus. We have demonstrated that the changes R1 and R2/R1 values are MMP9 concentration dependent in tumors. The combination of PMPSD with laser irradiation was more effective than single chemo-/photothermal therapy, and up-regulation of MMP9 in the tumor could eanhance the nanoplatform’s therapeutic effect.Purpose
In this study, we aimed to develop a theranostic nanoplatform with transformable magnetic resonance property for quantitative tumor bio-targeted markers and synergetic chemo-photothermal therapy.Methods
The magnetic nanocluster (PMP@USPIO/DOX), which is formed by self-assembly of an amphiphilic polymer (PMP)containing MMP9 substrate peptide wrapping with USPIO and DOX. The R1 and R2 values of PMPSD incubated with MMP9 were measured to verify the response of PMPSD to different MMP9 concentrations. The cellular uptake of the nanoparticles by 4T1 cells with high expression of MMP9 was observed by fluorescence microscopy. To examine the tumor derived MMP9 responsiveness of PMPSD, PMPSD was incubated in 4T1 cells and HK2 cells with low expression for 4 h, and then the MRI was performed. To further verify the activity of MMP9 in 4T1 cells, a MMP9 agonist and inhibitor were separately added to 4T1 cells for 4 h, then added PMPSD and incubated for 4 h again. The 4T1 subcutaneous tumor model of female BALB/ c nude mice was constructed, and the photothermal properties of the nanoplatform in vivo were verified by photothermal imaging. BALB/c mice bearing 4T1 tumor were scanned before and after injection of the PEG-SPIO, PMP@USPIO, and PMP@USPIO/DOX via tail vein at different time points of 0, 1, 4 and 12 h. In order to further confirm the response of MMP9 to PMPSD, and to evaluate the correlation between tumor signal changes and MMP9 levels in vivoTo further evaluate the photothermal and chemotherapeutic efficacy of PMPSD, tumor-bearing mice were divided into seven groups Tumor volumes, body weight, and CCR% of each group were measured over time after treatment. After treatment, tumors of each group were collected for Caspase3 immunohistochemical examination.Results
The amphiphilic polymer was successfully synthesized. TEM showed that the USPIOs were evenly and tightly arranged within the spherical core, and the hydration particle size of PMPSD was around 102.2 nm by DLS. In physiological conditions, the r2 value of the nanoplatform was 106.32mm-1s-1 which showed T2 effect. However, the r2 value decreased under MMP9 condition, and the r1 value increased from 4.52 mm-1s-1 to 6.39 mm-1s-1, proved that MMP9 induced the T2-T1 transform. The fluorescence signal can be activated specifically by MMP9. The nanoplatform had excellent stability , good photothermal stability, and possessed dual stimuli-responsive drug release properties. R1 values are positively correlated with MMP9 concentration. PMPSD could be uptaken by 4T1 cells after incubation for 3 h. The T1 signal of 4T1 cells was activated specially, and the MMP9 agonist group showed the highest T1 signal intensity and T1 relaxation rate while the inhibitor group exhibited the lowest T2 SNR and T2 relaxation rate, indicating that the high specificity and sensitivity of PMPSD in response to MMP9 and MMP9 activity could be evaluated by MR at cellular level. The nanoplatform had good responsiveness to MMP9 in vivo, and can achieve T2-T1 dual-mode imaging and non-invasive real-time visualization of drug release. The changes of MR signal (R1,R2/R1) in tumor were linearly correlated with the concentration of MMP9 after injection PMPSD for 4 h, indicating that the PMPSD nanoplatform is potentially useful to quantitatively analyze MMP9 in a non-invasive manner in vivo. PMPSD had the best photothermal effect compared to PEG-SPIO and PMPS. The PMPSD combined with irradiation treatment generated a significant inhibitory effect on tumor growth. Further, the treatment of PMPSD plus MMP9 with irradiation achieved the best antitumor efficacy. PMPSD had nontoxicity and few side effects on other organs.Conclusion
we reported a novel nanoplatform-based MMP9-responsive T2–T1 switching MRI contrast agent, which can be used not only for non-invasive visualization and quantitative analysis of MMP9 activity, but also as a carrier in photothermal sensitization chemotherapy. USPIO and doxorubicin accumulated into clusters through hydrophobic interactions in the hydrophobic region of amphiphilic polymers. The nanoplatform acts as a T2 contrast agent in physiological conditions due to the USPIO aggregation state, whereas in the tumor microenvironment, the nanoclusters are dissociated with tumor-derived MMP9, thus transforming the nanoplatform into a T1 contrast agent. We have demonstrated that the changes in tumor R1 and R2/R1 values are linearly correlated with MMP9 concentration and can indirectly identify MMP9 activity in tumors. Simultaneously, PMPSD exhibits a good photothermal effect. The combination of PMPSD with laser irradiation was more effective than single chemo-/photothermal therapy, and up-regulation of MMP9 in the tumor could enhance the nanoplatform’s therapeutic effect. Therefore, PMPSD could be applied as a versatile nanoplatform that can enable T1-T2 bimodal imaging, quantification of biological targets, monitoring drug release, and can be utilized for sensitization chemotherapy.Acknowledgements
The authors would like to acknowledge Shanghai General Hospital of Nanjing Medical University. This work was supported by National Natural Science Foundation of China (No. 81971664), Shanghai Pujang Program (2019PJD044).References
[1] Zhou Z, Bai R, Munasinghe J, Shen Z, Nie L, Chen X. T1–T2 Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents [J]. ACS Nano, 2017, 11(6): 5227-32.
[2] Lu H, Xu Y, Qiao R, Lu Z, Wang P, Zhang X, Chen A, Zou L, Wang Z. A novel clustered SPIO nanoplatform with enhanced magnetic resonance T2 relaxation rate for micro-tumor detection and photothermal synergistic therapy [J]. Nano Research, 2020, 13(8): 2216-25.
[3] Zhang W, Liu L, Chen H, Hu K, Delahunty I, Gao S, Xie J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents [J]. Theranostics, 2018, 8(9): 2521-48.
[4] Wang S, Zhou Z, Wang Z, Liu Y, Jacobson O, Shen Z, Fu X, Chen Z Y, Chen X. Gadolinium Metallofullerene-Based Activatable Contrast Agent for Tumor Signal Amplification and Monitoring of Drug Release [J]. Small, 2019, 15(16): e1900691.
[5] Cao Y, Mao Z, He Y, Kuang Y, Liu M, Zhou Y, Zhang Y, Pei R. Extremely Small Iron Oxide Nanoparticle-Encapsulated Nanogels as a Glutathione-Responsive T1 Contrast Agent for Tumor-Targeted Magnetic Resonance Imaging [J]. ACS applied materials & interfaces, 2020, 12(24): 26973-81.
[6] Lee N, Yoo D, Ling D, Cho M H, Hyeon T, Cheon J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy [J]. Chemical reviews, 2015, 115(19): 10637-89.
[7] Yi Z, Luo Z, Barth N D, Meng X, Liu H, Bu W, All A, Vendrell M, Liu X. In Vivo Tumor Visualization through MRI Off-On Switching of NaGdF4 -CaCO3 Nanoconjugates [J]. Advanced materials, 2019, 31(37): e1901851.
[8] Li J, Wang S, Wu C, Dai Y, Hou P, Han C, Xu K. Activatable molecular MRI nanoprobe for tumor cell imaging based on gadolinium oxide and iron oxide nanoparticle [J]. Biosensors and Bioelectronics, 2016, 86: 1047-53.
[9] Bai C, Jia Z, Song L, Zhang W, Chen Y, Zang F, Ma M, Gu N, Zhang Y. Time-Dependent T1-T2 Switchable Magnetic Resonance Imaging Realized by c(RGDyK) Modified Ultrasmall Fe3O4 Nanoprobes [J]. Adv Funct Mater, 2018, 28(32): 1802281.
[10] Lin J, Xin P, An L, Xu Y, Tao C, Tian Q, Zhou Z, Hu B, Yang S. Fe3O4–ZIF-8 assemblies as pH and glutathione responsive T2–T1 switching magnetic resonance imaging contrast agent for sensitive tumor imaging in vivo [J]. Chemical Communications, 2019, 55(4): 478-81.
[11] Cai Z, Wu C, Yang L, Wang D, Ai H. Assembly-Controlled Magnetic Nanoparticle Clusters as MRI Contrast Agents [J]. ACS Biomaterials Science & Engineering, 2020, 6(5): 2533-42.
[12] Wang Z, Xue X, Lu H, He Y, Lu Z, Chen Z, Yuan Y, Tang N, Dreyer C A, Quigley L, Curro N, Lam K S, Walton J H, Lin T Y, Louie A Y, Gilbert D A, Liu K, Ferrara K W, Li Y. Two-way magnetic resonance tuning and enhanced subtraction imaging for non-invasive and quantitative biological imaging [J]. Nature nanotechnology, 2020, 15(6): 482-90.
[13] Edward M, Quinn J A, Mukherjee S, Jensen M B, Jardine A G, Mark P B, Burden A D. Gadodiamide contrast agent 'activates' fibroblasts: a possible cause of nephrogenic systemic fibrosis [J]. The Journal of pathology, 2008, 214(5): 584-93.
[14] Kim B H, Lee N, Kim H, An K, Park Y I, Choi Y, Shin K, Lee Y, Kwon S G, Na H B, Park J G, Ahn T Y, Kim Y W, Moon W K, Choi S H, Hyeon T. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents [J]. J Am Chem Soc, 2011, 133(32): 12624-31.
[15] Vallabani N V S, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics [J]. 3 Biotech, 2018, 8(6): 279.
[16] Wang Z, Qiao R, Tang N, Lu Z, Wang H, Zhang Z, Xue X, Huang Z, Zhang S, Zhang G, Li Y. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer [J]. Biomaterials, 2017, 127: 25-35.
[17] Kim M H, Son H Y, Kim G Y, Park K, Huh Y M, Haam S. Redoxable heteronanocrystals functioning magnetic relaxation switch for activatable T1 and T2 dual-mode magnetic resonance imaging [J]. Biomaterials, 2016, 101: 121-30.
[18] Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y, Yu H, Gan Y, Wang Y, Mei L, Chen H, Hu H, Zhang Z, Jin Y. Recent progress in drug delivery [J]. Acta pharmaceutica Sinica B, 2019, 9(6): 1145-62.
[19] Kaittanis C, Shaffer T M, Ogirala A, Santra S, Perez J M, Chiosis G, Li Y, Josephson L, Grimm J. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching [J]. Nature communications, 2014, 5: 3384.
[20] Zhang Z T, Huang-Fu M Y, Xu W H, Han M. Stimulus-responsive nanoscale delivery systems triggered by the enzymes in the tumor microenvironment [J]. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV, 2019, 137: 122-30.
[21] Kang Z, Ding G, Meng Z, Meng Q. The rational design of cell-penetrating peptides for application in delivery systems [J]. Peptides, 2019, 121: 170149.
[22] Gulzar A, Xu J, Wang C, He F, Yang D, Gai S, Yang P, Lin J, Jin D, Xing B. Tumour microenvironment responsive nanoconstructs for cancer theranostic [J]. Nano Today, 2019, 26: 16-56.
[23] Lu J, Sun J, Li F, Wang J, Liu J, Kim D, Fan C, Hyeon T, Ling D. Highly Sensitive Diagnosis of Small Hepatocellular Carcinoma Using pH-Responsive Iron Oxide Nanocluster Assemblies [J]. Journal of the American Chemical Society, 2018, 140(32): 10071-4.
[24] Cao Z, Li W, Liu R, Li X, Li H, Liu L, Chen Y, Lv C, Liu Y. pH- and enzyme-triggered drug release as an important process in the design of anti-tumor drug delivery systems [J]. Biomedicine & Pharmacotherapy, 2019, 118: 109340.
[25] Yao Q, Kou L, Tu Y, Zhu L. MMP-Responsive 'Smart' Drug Delivery and Tumor Targeting [J]. Trends in pharmacological sciences, 2018, 39(8): 766-81.
[26] Synak A, Serdiuk I E, Grobelna B, Fudala R, Gryczynski I, Bojarski P. Spectroscopic method for estimation of MMP-9 enzyme concentration and activity [J]. Journal of Molecular Liquids, 2019, 286: 110936.
[27] Hoikkala S, Pääkkö P, Soini Y, Mäkitaro R, Kinnula V, Turpeenniemi-Hujanen T. Tissue MMP-2/TIMP-2-complex are better prognostic factors than serum MMP-2, MMP-9 or TIMP-1 in Stage I–III lung carcinoma [J]. Cancer Letters, 2006, 236(1): 125-32.
[28] Sienel W, Hellers J, Morresi-Hauf A, Lichtinghagen R, Mutschler W, Jochum M, Klein C, Passlick B, Pantel K. Prognostic impact of matrix metalloproteinase-9 in operable non-small cell lung cancer [J]. International journal of cancer, 2003, 103(5): 647-51.
[29] Jiang J, Shen N, Ci T, Tang Z, Gu Z, Li G, Chen X. Combretastatin A4 Nanodrug-Induced MMP9 Amplification Boosts Tumor-Selective Release of Doxorubicin Prodrug [J]. Advanced materials, 2019, 31(44): e1904278.
[30] Ansari C, Tikhomirov G A, Hong S H, Falconer R A, Loadman P M, Gill J H, Castaneda R, Hazard F K, Tong L, Lenkov O D, Felsher D W, Rao J, Daldrup-Link H E. Development of Novel Tumor-Targeted Theranostic Nanoparticles Activated by Membrane-Type Matrix Metalloproteinases for Combined Cancer Magnetic Resonance Imaging and Therapy [J]. Small, 2014, 10(3): 566-75.
[31] Juan Gallo N K, Ioannis Lavdas, Elizabeth Stevens, Quang-De Nguyen,, Marzena Wylezinska-Arridge E O A, * and Nicholas J. Long*. CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced magnetic resonance imaging.pdf [J]. Angewandte Communications, 2014.
[32] Yao Y, Cheng K, Cheng Z. Evaluation of a smart activatable MRI nanoprobe to target matrix metalloproteinases in the early-stages of abdominal aortic aneurysms [J]. Nanomedicine : nanotechnology, biology, and medicine, 2020, 26: 102177.
[33] Ali A, Zafar H, Zia M, Ul Haq I, Phull A R, Ali J S, Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles [J]. Nanotechnology, science and applications, 2016, 9: 49-67.
[34] Wang Z, Xue X, He Y, Lu Z, Jia B, Wu H, Yuan Y, Huang Y, Wang H, Lu H, Lam K S, Lin T Y, Li Y. Novel redox-responsive polymeric magnetosomes with tunable magnetic resonance property for in vivo drug release visualization and dual-modal cancer therapy [J]. Adv Funct Mater, 2018, 28(33).
[35] Zhou Z, Tian R, Wang Z, Yang Z, Liu Y, Liu G, Wang R, Gao J, Song J, Nie L, Chen X. Artificial local magnetic field inhomogeneity enhances T2 relaxivity [J]. Nature communications, 2017, 8: 15468. [36] Cho H J S Y H, Nam K S . Ginkgolide C Inhibits Platelet Aggregation in cAMP- and cGMPDependent Manner by Activating MMP-9 [J]. Biological & Pharmaceutical Bulletin, 2007, 30(12): 2344.
[37] Xu K, Ma C, Xu L, Ran J, Jiang L, He Y, Adel Abdo Moqbel S, Wang Z, Wu L. Polygalacic acid inhibits MMPs expression and osteoarthritis via Wnt/beta-catenin and MAPK signal pathways suppression [J]. International immunopharmacology, 2018, 63: 246-52.
[38] Bulte J, Douglas T, Witwer B, Zhang S C, Strable E, Lewis B K, Zywicke H, Miller B, Gelderen P V, Moskowitz B M. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells [J]. Nature Biotechnology, 2001, 19(12): 1141-7.