4885
High Longitudinal and Transverse Relaxivities with Rare Earth ions doped Mn0.6Zn0.4Fe2O4: a Potential for dual-mode Contrast Agents1Division of Medical Physics, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 2Faculty of Physics, University of Isfahan, Isfahan, Iran (Islamic Republic of)
Synopsis
Keywords: Contrast Mechanisms, Contrast Agent, Relaxometery, longitudinal Relaxation Time
MRI contrast agents have gained extensive attention for providing contrast between normal and abnormal tissue. However, single-mode contrast agents do not always provide the required contrast enhancement for disease detection. One effective strategy for avoiding ambiguous images is to design MRI contrast agents with simultaneous T1 and T2 shortening effects. The purpose of this work is to synthesize rare earth ions doped Mn-Zn ferrite (ReMZF) nanoparticles and characterize them with a 1.5T MRI scanner in vitro. The ReMZF exhibited high r1 and r2 relaxivities but moderate r2/r1 which make them suitable to be used as dual-mode contrast agents.
Introduction
MRI contrast agents are indispensable tools for many clinical applications such as tumor detection, MR angiography or perfusion measurements1. In general, MRI contrast agents improve the sensibility and reliability of MRI by shortening the water relaxation times2: Gd (III) and other paramagnetic metal ions chelates are widely applied as T1-weighted contrast agents3, whereas iron oxide4 and spinel ferrite like Mn-Zn ferrite (MZF)5 nanoparticles locally shorten T2. Tunable contrast agents are desirable to enhance either the T1- or the T2-weighting6. Doping of iron-based T2-weighted contrast agents with rare earth ions could be an alternative to ordinary dual-mode contrast agents. In this work, we investigated the effect of doping different rare earth ions (Eu3+, Gd3+, Sm3+, Y3+ and Tb3+) on r1 and r2 relaxivities of Mn-Zn spinel ferrite.Methods
Rare earth-doped Mn-Zn ferrite samples (ReMZF) were prepared by dissolving adequate amounts of Fe3+, Re3+, Mn2+, Zn2+ salts with a molar ratio of Fe:Eu equals 10 and citric acid in water on a hot magnetic stirrer at 80 °C. A solution of 2M NaOH was added to the salt solution until the pH was between10 to 12. The brown precipitation was transferred to an autoclave and kept under 180 °C temperature in an oven. The resulting black precipitation was washed with water and dried at 60 °C for 4 hours to obtain nanopowders of ReMZF. The crystal structure of nanoparticles was analyzed by X-Ray Diffraction (XRD) method. Magnetic properties were investigated via the VSM method. Relaxometry measurements were at 1.5T performed using a clinical Magnetom Aera MRI system (Siemens, Germany) equipped with a 15-channel transmit/receive knee coil (QED, Cleveland, OH). T1 relaxation measurements were conducted using a Turbo FLASH pulse sequence7 with TR=4.6ms, TE=2ms and different saturation recovery delays TS = 100, 200, 300, 400, 500, 750, 1250, 2500, and 5000ms. Finally, T1 was obtained by fitting the experimental signal data to the saturation recovery equation:$$S(TS)=S_0(1-e^{-TS/T1})$$
To measure the transverse relaxation time T2, a multi-echo spin echo pulse sequence with a Carr-Purcell-Meiboom-Gill (CPMG)8 scheme was used with 32 different spin echoes between TE1 = 8 ms and TE32 = 256 ms. Then the transverse relaxation times (T2) were estimated by fitting experimental signal data to the following equation:
$$S(TE)=S_0e^{-TE/T2}$$
From the relaxation times, the r1 and r2 relaxivities were calculated as a function of the contrast agent concentration C:
$$R_i=1/T_i=1/T_{io}+1/T_i^{CA}=R_{io}+r_i$$
with $$$i=1,2$$$. Here, $$$T_{io}=R_{io}^{-1}$$$ denotes the relaxation time of the solvent in the absence of the contrast agent.
Results
Figure 1 exhibited that all the XRD patterns matched a cubic spinel phase (JCPDS file N° 96-200-9104) with no trace of any secondary phase except for the TbMZF sample which contains extra peaks of Tb2O3 phase.Table 1 shows that doping rare earth ions has decreased the saturation magnetization (Ms) of the MZF. The Ms value for MZF is 56 emu/g while for GdMZF, EuMZF, SmMZF, YMZF and TbMZF are 44, 45, 52, 48, and 43 emu/g respectively.
The T1- and T2-weighted MR images of ReMZF samples were illustrated in Figure 2. With increasing the concentration, the signal improvement in the T1-weighted images, and signal reduction in T2-weighted images for the EuMZF, GdMZF and YMZf samples are more significant than those for the TbMZF and SmMZF. Figure 3a and 3b illustrate the r1 and r2 relaxivities curves for ReMZF, and the r1 and r2 values are summarized in Table 2. The GdMZF sample has the highest r1 of 28s-1mM-1 and the TbMZF sample has a very low r1 of 1.36s-1mM-1, and r2 is 481 s-1mM-1 for YMZF and 63 s-1mM-1 for TbMZF. Our results indicate that GdMZF, EuMZF, and YMZF are excellent candidates for T1-weighted and T2-weighted contrast agents.
Discussion and Conclusion
There is a direct relationship between the reduction in saturation magnetization of MZF and the magnitude of rare earth ion's magnetic moment. With larger magnetic moments a higher reduction in saturation magnetization is achieved. The MZF crystal structure has two sublattices A and B with a net magnetization of M = MB-MA. Rare earth ions prefer to enter the A sublattice9; thus, doping with these ions increases MA and in total decreases M. Among the ReMZF samples, GdMZF had the greatest r1 relaxivity due to the simultaneous presence of high spin paramagnetic ions of Gd3+ with 7 unpaired electrons (high magnetic moment of 7.94µB) and Mn2+ with 5 unpaired electrons (µ=5.9µB) in its structure. The shielding effect of paramagnetic ions around the water protons decreases the magnetic field sensed by protons and, consequently, causes water protons to resonate at lower frequencies (chemical shift effect). With decreasing Larmor frequency the frequencies of the water protons associated with the molecular motion in the surrounding medium can match better, leading to an efficient transfer of energy and shorter T1 (larger r1). The measured values of r2/r1 suggest that YMZF with high r2 and r2/r1 values is an excellent T2 contrast agent. On the contrary, GdMZF and EuMZF can be applied as dual-mode contrast agents due to high r1 and r2 but moderate r2/r1.Acknowledgements
No acknowledgement found.References
1. Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int. J. Nanomedicine 2015; 10:1727–1741 doi: 10.2147/IJN.S76501.
2. Avasthi A, Caro C, Pozo‑Torres E, Leal MP, García‑Martín ML. Magnetic Nanoparticles as MRI Contrast Agents. In: Puente-Santiago AR, Rodríguez-Padrón D, editors. Surface-modified Nanobiomaterials for Electrochemical and Biomedicine Applications. Topics in Current Chemistry Collections. Cham: Springer International Publishing; 2020. pp. 49–91. doi: 10.1007/978-3-030-55502-3_3.
3. Zhou Z, Lu Z-R. Gadolinium-Based Contrast Agents for MR Cancer Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013; 5:1–18 doi: 10.1002/wnan.1198.
4. Neuwelt A, Sidhu N, Hu C-AA, Mlady G, Eberhardt SC, Sillerud LO. Iron-Based Superparamagnetic Nanoparticle Contrast Agents for MRI of Infection and Inflammation. AJR Am. J. Roentgenol. 2015;204: W302–W313 doi: 10.2214/AJR.14.12733.
5. Shultz MD, Calvin S, Fatouros PP, Morrison SA, Carpenter EE. Enhanced ferrite nanoparticles as MRI contrast agents. J. Magn. Magn. Mater. 2007; 311:464–468 doi: 10.1016/j.jmmm.2006.10.1188.
6. Park JC, Lee GT, Kim H-K, et al. Surface Design of Eu-Doped Iron Oxide Nanoparticles for Tuning the Magnetic Relaxivity. ACS Appl. Mater. Interfaces 2018.
7. Bock M, Schulz J, Ueltzhoeffer S, Giesel F, Voth M, Essig M. Intravascular contrast agent T1 shortening: fast T1 relaxometry in a carotid volunteer study. Magma N. Y. N 2008; 21:363–368 doi: 10.1007/s10334-008-0134-2.
8. Meiboom S, Gill D. Modified Spin‐Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958; 29:688–691 doi: 10.1063/1.1716296.
9. Lin M, Huang J, Sha M. Recent Advances in Nanosized Mn-Zn Ferrite Magnetic Fluid Hyperthermia for Cancer Treatment. J. Nanosci. Nanotechnol. 2014; 14:792–802 doi: 10.1166/jnn.2014.9135.
Figures
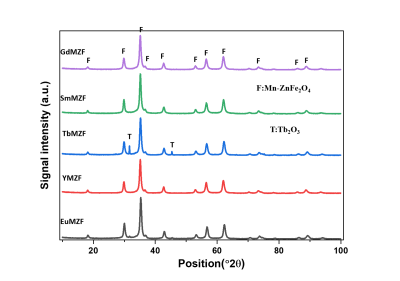
Figure 1. XRD patterns of ReMZF samples.
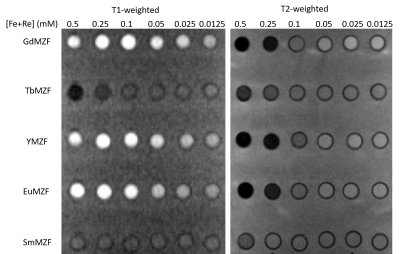
Figure 2. T1- and T2-weighted images of ReMZF samples.
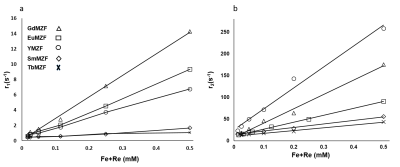
Figure 3. The variation of the relaxation rates (a) R1 and (b) R2 as a function of concentration for ReMZF samples at 1.5T.
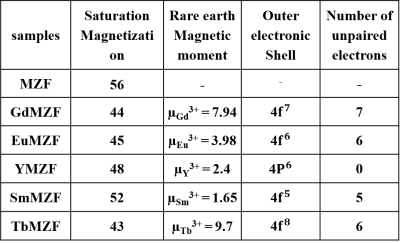

Table 2: r1, r2 and r2/r1 of ReMZF samples at 1.5T.