4884
Engineering novel functional brain imaging contrast agents via iron oxide encapsulation in human red blood cells1Biomolecular Sciences, University of Urbino Carlo Bo, Urbino, Italy, 2Centre for Hyperpolarisation in Magnetic Resonance (CHyM), Chemistry, University of York, York, United Kingdom, 3Psychology, University of York, York, United Kingdom, 4Sport and Exercise Science, Institute of Science, Manchester Metropolitan University, Manchester, United Kingdom
Synopsis
Keywords: Contrast Agent, Contrast Agent, red blood cells, Super Paramagnetic Iron Oxide Nanoparticles
We have developed a next generation, safe, personalised iron-based contrast agent (CA) for empowering functional Magnetic Resonance Imaging (fMRI). Our proprietary method uses state-of-the-art red blood cell (RBC) encapsulation technology to engineer biocompatible super-paramagnetic CAs able to withstand rapid clearance from the bloodstream by macrophages (as part of the mononuclear phagocytic/reticuloendothelial system). The agent is validated in a multi-modal (concurrent fMRI and intrinsic optical imaging) preclinical rat model as a novel CA for the mapping of cerebral blood volume (CBV) in response to somatosensory stimulation, providing a non-BOLD alternative for accurate, high-resolution mapping of brain function.Introduction
In the field of cognitive neuroscience, measures of acute changes in Cerebral Blood Volume (CBV) and Flow (CBF) demonstrate significant advantages over routine Blood Oxygenation Level Dependent (BOLD) functional Magnetic Resonance Imaging (fMRI) signals1-4. Furthermore, non-BOLD fMRI offers layer specific high-resolution mapping of brain function1,3. An overwhelming range of non-BOLD techniques now exist (e.g., GRASE, ASL, SSFP, functional diffusion, phase-sensitive fMRI, VASO, etc). Unfortunately, these alternatives offer lower contrast-to-noise (CNR), are of unknown signal source5-6, or are simply not as easy to implement as gradient-echo based BOLD fMRI. Hence, clinical uptake of these important non-BOLD fMRI approaches remains limited. New technology permitting easier targeting of CBV markers, with higher contrast will reverse this situation.Preclinical fMRI data demonstrates that the high CNR signal proffered through use of intravenous iron oxide-based contrast agents delivers functional sensitivity at cortical laminar resolutions7-10. Unfortunately, due to short half-life, clinical delivery of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) for fMRI remains to be realised.
Using red blood cell (RBC) encapsulation technology (Fig.1A), we have engineered a personalised blood pool SPION contrast agent with a much longer blood retention time11-12. RBCs as carriers have the advantages that they are biocompatible, completely biodegradable and can be easily handled. This approach permits high CNR and longitudinal assessment of brain function. Here we validate this novel CA using concurrent fMRI and 2D-intrinsic optical imaging (Fig 1B) methods to measure neuronal responses to somatosensory stimulation.
Methods
Preparation: Ferucarbotran contrast agent (Meito Sangyo) was encapsulated into human RBCs (using 11.2 mg Fe/ml of 70% haematocrit loaded solution). The process involved hypotonic dialysis, isotonic resealing and re-annealing (Figure 1A)13. The T1 longitudinal relaxation time of the Ferucarbotran-loaded-RBCs at 44% haematocrit were measured at 9.4T (Avance-400 NMR, Bruker) to determine iron concentration. Transmission Electron Microscopy (TEM) was used to confirm entrapment of the SPION within the RBCs and to evaluate morphological changes. 1.5 ml of human Ferucarbotran-loaded-RBCs at 44% haematocrit were used for injection into non-recovery rat models.Imaging: Pre-clinical MRI measurements were made at 7 Tesla (Bruker BioSpec 70/30, 310mm bore). Urethane anaesthetized rats were artificially ventilated and cannulated for monitoring arterial blood pressure and intravenous infusion. A thinned skull cranial window allowed direct optical imaging of the cortex (see below). fMRI data were acquired in the coronal plane with a GE-EPI readout (TR/TE=1000/12ms, 64*64, FOV=30mm, slice thickness=2mm, fa 90°). BOLD (pre-CA injection) and CBV (post-CA injection) signal changes to whisker stimulation (16s, 1.2mA, 5Hz) and/or respiratory challenge (increased end-tidal FiCO2 <10%) were obtained concurrently with optical measurements of total hematocrit (HbT) and oxygenation (Y) changes using an MR compatible endoscope (Fig.1B).
Following BOLD fMRI measurements to somatosensory stimulation injection of the encapsulated cells (1ml) is completed in 0.1ml stages. Structural gradient echo images (256*256, FOV 30mm, 9 slices, 1mm thick) are captured for assessment of baseline blood volume fraction14 to aid quantification of CBV-fMRI measures. Post experiment animals were sacrificed with brain, spleen, liver and kidney tissue frozen and stored for later ICP-MS analysis. Blood samples were used to assess magnetic susceptibility following the methods of Tropres et.al. (2001).
The optical endoscope assembly incorporated a 2.0 cm diameter surface coil fixed to the head around the cranial window to form a well. Filling the well with deuterium oxide avoided air-tissue susceptibility artifacts around the cranial window. 2D-OIS used a Lambda DG-4 switching Galvanometer (Sutter Instruments) with four λ (495, 586, 559 and 575nm). CCD frame rate was 32Hz, giving an 8Hz effective frame rate per λ. Spectral analysis used an MR based heterogeneous tissue model and produced 2D time resolved images of oxy (HbO2), deoxy (Hbr) and total haemoglobin changes.
Results
TEM confirmed the presence of iron oxide monodispersed into cells and no cell morphological changes (Fig.2). NMR confirmed SPION concentration encapsulated in the RBCs between 5.6 – 11.2 mM Fe. These values were obtained using the r1-value (1.3003) calculated from the calibration curve specifically obtained for Ferucarbotran and human RBCs11 and their T1 measurements (Table 1). Haemocytometer measures of the Ferucarbotran-RBC constructs such as MCV, MCH and MCHC were similar to non-dialysed (ND) and unloaded (UL) RBCs (Table.1).Control experiments confirmed that physiological parameters (breathing rate, temperature, BP etc) of the rat were not affected by the injection of human RBCs (non-dialysed).
Preliminary data was obtained following injection of 1.5 ml of Ferucarbotran-loaded RBCs to i) estimate baseline blood volume fractions in rat brain and ii) validate CBV weighting of fMRI signals. Data here were compared to experiments following injection of free Ferucarbotran only (Fig.3).
Conclusions
Here we explore the use of a new iron-based contrast agent utilising SPION encapsulation into RBCs. We demonstrated successful encapsulation into human cells. We tested whether this approach permits high contrast CBV weighted fMRI measurements in a preclinical rat model. CBV weighting of the new CA was validated using bespoke concurrent fMRI and optical spectroscopy methodologies. Measures were directly compared to free Ferucarbotran based data.Acknowledgements
This project was financed by PON BIO-D Development of diagnostic biomarkers for precision medicine and personalized therapy. Project Code: ARS01_00876 (CUP B32F20000270005)References
1 Huber, L. Uludag, K., et al. Non-BOLD contrast for laminar fMRI in humans: CBF, CBV, and CMRO2. Neuroimage, 2019;197: 742-60
2 Chu, K., Xu, Y., et al. Bulk magnetic susceptibility shifts in nmr studies of compartmentalized samples: use of paramagnetic reagents. Magn Reson Med., 1990; 13(2):239–62
3 Fracasso, A., Luijten, P. R., et al. Laminar imaging of positive and negative BOLD in human visual cortex at 7 T. NeuroImage, 2018; 164:100–11.
4 Gagnon, L., Sakadzic, S., et al. Quantifying the Microvascular Origin of BOLD-fMRI from First Principles with Two-Photon Microscopy and an Oxygen-Sensitive Nanoprobe. J Neurosci, 2015; 8(3-4):307–10.
5 Huber, L., Poser, B.A., et al. Validating layer-specific VASO across species. Neuroimage, 2021; 237:118-195
6 Kuriwa, D., Obata, T., et al. Signal contributions to heavily diffusion-weighted functional magnetic resonance imaging investigated with multi-SE-EPI acquisitions. NeuroImage, 2014;98:258-65.
7 Kennerley, A. J., Mayhew, J. E., et al. Vascular Origins of BOLD and CBV fMRI Signals: Statistical Mapping and Histological Sections Compared. Open Neuroimaging J., 2010; 4:1–8.
8 Van Zijl, P. C., Eleff, S. M., et al. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med., 1998; 4:159-67
9 Mandeville, J. B., Marota, J. J., et al. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J. Cereb. Blood Flow Metab., 1999; 19(6):679–89
10 Mandeville, J.B. IRON fMRI measurements of CBV and implications for BOLD signal, Neuroimage, 2012 62(2): 1000-8
11 Antonelli, A., Sfara, C., et al. New Strategies to Prolong the In Vivo Life Span of Iron- Based Contrast Agents for MRI. PLOS One. 2013; 8(10) e78542
12 Antonelli, A., Sfara, C., et al. Characterization of ferucarbotran-loaded RBCs as long circulating magnetic contrast agents. Nanomedicine., 2016; 11(21):2781-2795
13 Antonelli, A., Sfara, C., et al. New Biomimetic Constructs for Improved In Vivo Circulation of Superparamagnetic Nanoparticles. J. Nanosci. Nanotechnol., 2018; 8 (5) 2270-2278(9)
14 Tropes, I., Grimault, S., et al. Vessel Size Imaging, Magn Reson Med., 2001; 45:397–408
Figures
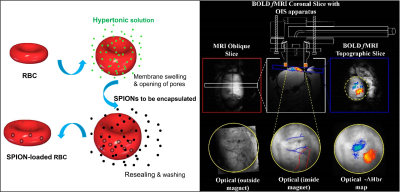
Figure 1. Schematic representation of the SPIONs loading into RBCs by hypotonic dialysis, isotonic resealing and re-annealing (A). Concurrent fMRI and optical spectroscopy methods permit multi-parametric measure of the same neuronal event and direct validation of the contrast induced by our novel CA(B).
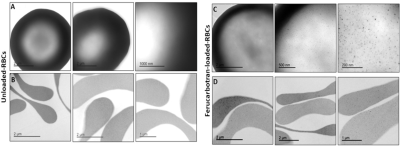
Figure 2. Transmission Electron Microscopy (TEM) analysis of unloaded RBCs (A. whole cell and B. cross section slice) and Ferucarbotran-loaded-RBCs (C. whole cell and D. cross section slice). Monodispersed iron oxide nanoparticles inside the cells are observable.
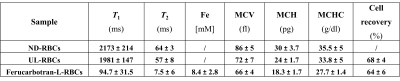
Table 1: NMR measurements of T1 and T2 of Ferucarbotran-loaded RBCs and some of their biological parameters. ND-RBCs: Non dialysed RBCs; UL-RBCs: Unloaded RBCs were prepared with the same conditions of loaded RBC but in the absence of iron oxide; Ferucarbotran-L-RBCs: RBCs obtained using 11.2 mg Fe of Ferucarbotran. T1 = longitudinal relaxation time, T2 = transverse relaxation time, MCV: Mean corpuscular volume; MCH: Mean haemoglobin concentration; MCHC: Mean corpuscular haemoglobin concentration.
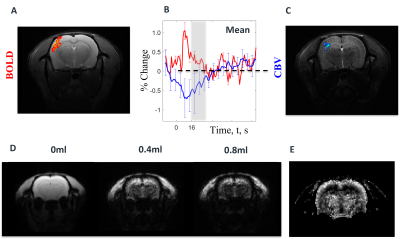
Figure 3: fMRI measures in response to somatosensory whisker stimulation (16s, 1.2mA, 5Hz). (A) Resultant BOLD signal map overlaid on a structural scan. (B) time series of BOLD and CBV (using Ferucarbotran) signal. (C) CBV map (post Ferucarbotran CA infusion) overlaid on a structural scan. (D) Structural Gradient Echo images during injection of Ferucarbotran and (E) resultant R2* blood volume fraction map.