4880
First Results of Novel Contrast Agent Based on Ultrasmall Paramagnetic Nanoparticles for T1-Weighted MRI
Khallil Taverna Chaim1, Mayara Klimuk Uchiyama1,2, Robson Raphael Guimaraes2, Koiti Araki2, and Claudia da Costa Leite 1
1School of Medicine - Radiology, University of Sao Paulo, Sao Paulo, Brazil, 2Institute of Chemistry, University of Sao Paulo, Sao Paulo, Brazil
1School of Medicine - Radiology, University of Sao Paulo, Sao Paulo, Brazil, 2Institute of Chemistry, University of Sao Paulo, Sao Paulo, Brazil
Synopsis
Keywords: Contrast Agent, Contrast Agent, nanoparticles
MRI is one of the best imaging techniques in clinical analysis because it is non-invasive and has high resolution. Contrast agents are used to further improve these images, as they increase the level of soft tissue detail, differentiating regions of interest. Currently, Gd-based contrast agents have important precautions. In this work we present a new nanoparticle based on iron oxide, with 4 nm and positive contrast (T1-weighted). Thus, effects similar to gadoteric acid, with reduced signal persistence and partial renal excretion, indicating great stability, this new paramagnetic nanoparticle has great potential to be applied as a contrast agent in MRI.Introduction
MRI contrast agents interfere with the relaxation processes by shortening longitudinal (or spin-lattice, T1) and transverse (or spin-spin, T2) relaxation times. Gadolinium (Gd) was been evaluated as the most effective paramagnetic ion for positive contrast in T1-weighted images. The administration of such Gd-based contrast agents (GBCAs) is associated, due to its toxicity, with the development of nephrogenic systemic fibrosis and accumulation of metals. Efforts are underway to find safer contrast agents with clinical applicability and performance comparable to GBCAs. Superparamagnetic iron oxide nanoparticles (SPIONs) are typical T2/T2* contrast agents, since their magnetic susceptibility increases local heterogeneities (high values of r2 and r2/r1), overlapping T1. In this way, the development of contrast agents based on ultra-small iron oxide nanoparticles (USIONs < 5 nm) allows them to behave as T1 contrast agents. Thus, a new class of ultra-small paramagnetic nanoparticles (UPNs) with adjustable magnetic contrast and magnetic resonance properties was realized by decorating biocompatible UPNs with adequate amounts of iron oxide (1,2,3).Methods
We coated our nanoparticles of amorphous diamagnetic metal oxides with paramagnetic metal ions bound on their surface, with 0.25 mM Fe and functionalized them with citrate. The formulation for biological application, with 150 mM propylene glycerol to adjust the osmolarity to 1600 mOsm/kg in H2O, was prepared, filtered through a 0.22 µm filter and sealed in 2 mL sterile amber glass ampoules (zeta potential of -34 .7 ± 5.5 mV). Agar preparations with concentrations of 0.1, 0.25, 5, 10, 15 and 20 mM of paramagnetic ion were used for T1 and T2 relaxometry in 3 and 7 Tesla. The new UPNs as a T1 contrast agent were evaluated in vivo using Wistar rats (4 males, 2 groups) in 7T. The protocol was approved by the FMUSP Animal Ethics Committee (nº 966/2018). Images were acquired before and after 20 seconds i.v. Bolus injection of gadoteric acid (GA) or UPN (0.1 mmol/kg body weight, 25 mM GA or Fe). The average of signal for 2 animals per group was plotted as a function of time and regions of interest was manually segmented. The baseline was corrected in relation to the signal before the injection of the Cas, without normalization.Results
The GA and novel UPNs relaxometry on agar phantom were compared. Its r1 and r2 relaxivities were determined to be 1.76 mM-1 s-1 and 2.43 mM-1 s-1 at 3 T, and 1.32 mM-1 s-1 and 2.35 mM-1 s-1 at 7 T, as compared to 7.32 mM-1 s-1 and 4.84 mM-1 s-1 at 3 T and 3.80 mM-1 s-1 and 3.53 mM-1 s-1 at 7 T of GA. Thus, the r2/r1 ratio for the UPN is 1.38 (3T) and 1.78 (7T), in comparison with 0.66 (3T) and 0.93 (7T) for GA, values typical of T1-contrast agents. The T1-weighted as a function of time obtained post-injection of the contrast agents on animals show the enhancement of the brightness in the heart, liver, blood vessels and kidneys, in comparison to the images obtained before the injection, as expected. The response of GA and UPN in enhancing the T1-signal intensity in heart and kidneys after injection of the contrast agents in rats shows similarity. A T1-weighted MR images obtained before and minutes after bolus injection of the contrast agents GA and UPN indicates the progressive accumulation of GA in the renal pelvis after 11 min of injection and the UPN also showed a tendency of renal accumulation and excretion, but took a much longer time (21 min) after the injection to start appearing in the kidneys. After 24h, no enhancing in T1-signal intensity was observed.Discussion
The novel UPNs exhibiting r2/r1 values lower than 2, thus enabling high quality images as using GBCAs, as demonstrated in agar phantom relaxometry. The curves for 5 mM paramagnetic ion in agar, at 7T, indicates that UPNs shorten T2 and T1 as well, but less effectively than GA. This is expected considering that Gd3+ has a larger number of unpaired electrons than Fe3+. Considering the in vivo study and the glomerular filtration barrier, with 5-6 nm molecular size cutoff, the partial urinary excretion was expected since UPNs are smaller than 5.5 nm. Furthermore, the contrast effect appears to last much longer for UPN than for GA, probably indicating a much longer circulation time, but without Gd. In the case of the heart, the signal increases until it peaks and then decreases, as expected due to recirculation. No enhancing of signal was observed after 24 hours, what is important for clinical applications and persistent with other SPIONs and USIONs (3,4,5,6).Conclusion
The novel type of ultrasmall paramagnetic nanoparticle designed as T1-weighted MR contrast agent presented relaxometric parameters comparable to the gadoteric acid in agar phantoms, as well as similar image quality after i.v. bolus injection in Wistar rats but longer effect than GA using a similar application protocol on first hours. After 24 hours of injection the signal return to pre-contrast conditions. Also, the renal elimination rate of UPN was about half of GA, suggesting a much longer circulation time and contrast effect, which can be advantageous as blood-pool contrast agent for MR angiography6 and blood-brain barrier disruption.Acknowledgements
The authors would like to thank the financial support granted by São Paulo Research Foundation (FAPESP, KA 2018/21489-1 and PISA 2009/54323-0), National Council for Scientific and Technological Development (CNPq, KA grant 442599/2019-6, 401581/2016-0, 303137/2016-9 and 402281/2013-6).References
- Jeon, M.; Halbert, M. v.; Stephen, Z. R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Advanced Materials 2021, 33 (23), 1906539. https://doi.org/10.1002/ADMA.201906539.
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F. C.; Grist, T. M.; Law, M.; Lee, J. M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; Prince, M. R.; Schild, H. H.; Weinreb, J. C.; Yoshikawa, K.; Pietsch, H. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Advances in Therapy 2016, 33, 1–28. https://doi.org/10.1007/S12325-015-0275-4
- Wei, H.; Bruns, O. T.; Kaul, M. G.; Hansen, E. C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; Cordero, J. M.; Heine, M.; Farrar, C. T.; Montana, D. M.; Adam, G.; Ittrich, H.; Jasanoff, A.; Nielsen, P.; Bawendi, M. G. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proceedings of the National Academy of Sciences of the United States of America 2017, 114 (9), 2325–2330. https://doi.org/10.1073/PNAS.1620145114.
- Du, B.; Yu, M.; Zheng, J. Transport and Interactions of Nanoparticles in the Kidneys. Nature Reviews Materials 2018, 3 (10), 358–374. https://doi.org/10.1038/s41578-018-0038-3.
- Adhipandito, C. F.; Cheung, S. H.; Lin, Y. H.; Wu, S. H. Atypical Renal Clearance of Nanoparticles Larger Than the Kidney Filtration Threshold. International Journal of Molecular Sciences 2021, 22 (20), 11182. https://doi.org/10.3390/IJMS222011182
- Wagner, M.; Wagner, S.; Schnorr, J.; Schellenberger, E.; Kivelitz, D.; Krug, L.; Dewey, M.; Laule, M.; Hamm, B.; Taupitz, M. Coronary MR Angiography Using Citrate-Coated Very Small Superparamagnetic Iron Oxide Particles as Blood-Pool Contrast Agent: Initial Experience in Humans. Journal of Magnetic Resonance Imaging 2011, 34 (4), 816–823. https://doi.org/10.1002/JMRI.22683
Figures
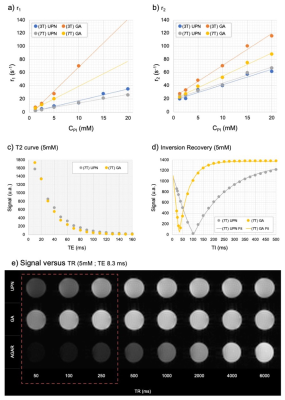
MR relaxometry of UPNs and GA. Comparison of (a) r1 and (b) r2 curves obtained at 3 T and 7 T magnetic fields in agar phantoms with increasing concentrations of the paramagnetic ions. (c) T2 and (d) Inversion Recovery curves at 5 mM of paramagnetic ion and 7 T magnetic field showing the shortening of T2 and T1, respectively. (e) Spin Echo images for UPN and GA in agar as compared to agar control acquired in different times of TR, emphasizing the hypersignal at low TRs (red dotted square). CPI: concentration of paramagnetic ion (mM).
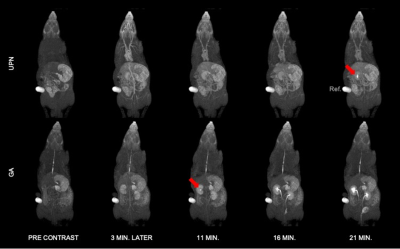
T1-weighted MR images of Wistar rats before (Pre-contrast) and after bolus injection of GA and UPN contrast agents. Red arrows show the arrival and accumulation of the contrast agent on the renal calyx at different times.

Average of T1-signal intensity of 2 rats as a function of time in the (top) heart and (bottom) kidneys, as indicated by the respective ROIs, after 20-second bolus injection of GA and UPN.
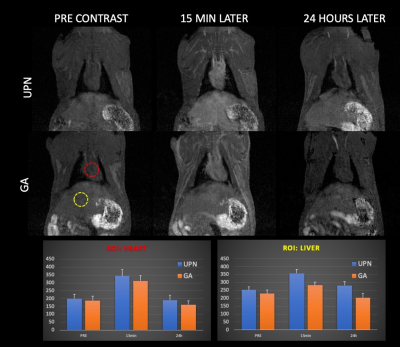
Average of T1-signal intensity as a function of time, pre injection and after injection (15 min and 24h later) of GA and UPN. The bar chart for ROIs on heart (left) and kidneys (right), indicated the return to pre-contrast conditions.
DOI: https://doi.org/10.58530/2023/4880