4879
Molecular Weight of Sugar Structures impacts Shielding Effect of Gd-Ions in Polysaccharides.1German Cancer-research center (DKFZ), Heidelberg, Germany, 2Charite Berlin, Berlin, Germany, 3Universität Heidelberg, Heidelberg, Germany
Synopsis
Keywords: Contrast Agent, Relaxometry, GBCA, gadolinium, long-term deposition, GAGs, dextran sulfate
Detailed biochemical information on the long-term deposition of Gd3+ in the body after administration of GBCAs is still missing. Glycosaminoglycans should be considered as chelators of released Gd3+. We used dextran-sulfate to study the effect of polysaccharides with different molecular weights on the observable relaxivity. Chelation was observed through an increase in relaxation. Moreover, a molecular weight-specific shielding revealed by subsequent reduced R1 occurs at higher polysaccharide/Gd-ion ratios. This coincided with observations of Gd-induced aggregation. Similar shielding of re-chelated Gd3+can lead to an underestimation of deposited Gd-ions in the body and requires further research.
Introduction:
Zinc and other divalent endogenous ions can compete for gadolinium-based contrast agent (GBCA) chelators in the body. The dissociation of Gd3+ via divalent ions can lead to a subsequent long-term deposition of Gd-ions in biological tissues [2-4]. A much-discussed topic is thereby the quest for involved interaction partners and the exact biochemical processes responsible for the long-term deposition [1-4]. An explanation for the frequently observed hyperintensities in various tissues could be caused by Gd-ion containing macromolecular structures[4-6]. Candidates for an interaction with Gd-ions are numerous, however glycosaminoglycans (GAGs) hold promising potential to be considered as key players[1,5,6]. Nevertheless, research on these gadolinium-sugar complexes is still in its infancy. In our study, a series of dextran sulfate solutions was used to investigate the influence of diverse molecular environments on the relaxivity of Gd-ions after the transchelation to polysaccharide structures with different molecular weights.Materials and Methods:
All MR measurements were performed on a 9.4 T preclinical MRI system (Bruker, Ettlingen, Germany). T1 measurements were performed using a dephasing recovery sequence consisting of 50 π/2 pulses with subsequent gradient spoiling and image acquisition. R1 values were calculated from ROI-averaged values from R1 maps. To model the transchelation processes of Gd-ions to different polysaccharides, CSA and dextran sulfate (5 kDa, 8 kDa, 20 kDa, 40 kDa, 500 kDa) in combination with GdCl3 were used as a model system. Light scattering measurements were used to investigate the increase of the aggregate size as a result of Gd-ion binding to dextran sulfate and CSA.Results:
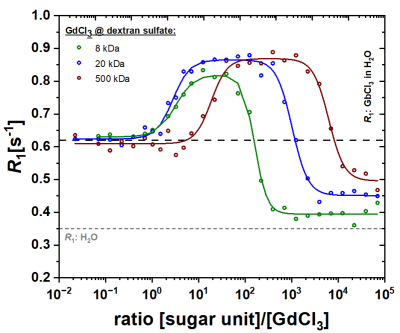
An increase in R1 was observed after the binding of Gd-ions to the added polysaccharides (Fig.1). Subsequently, a reduction of R1 could be observed with increasing polysaccharide concentration. Figure 1 shows R1 as a function of the concentration ratio between dextran sulfates with different molecular weight (adjusted to the total number of sugar units) and Gd-ions in solution. In all measurements, R1 increases from ~0.6 s-1 (R1 of 25 μM Gd3+ in H2O) to ~0.8 s-1 and to ~0.85 s-1 for dextran sulfates of MW = 5 kDa, 20 kDa and 500 kDa, respectively. The maxima are reached at ratios of ~101.1, ~101.3, and ~102.3. Subsequently, R1 decreases to ca. 0.4 s-1, 0.45 s-1, and 0.5 s-1 at ratios of ~103.1, ~104, and ~105 for dextran sulfates of MW = 5 kDa, 20 kDa and 500 kDa, respectively.Discussion:
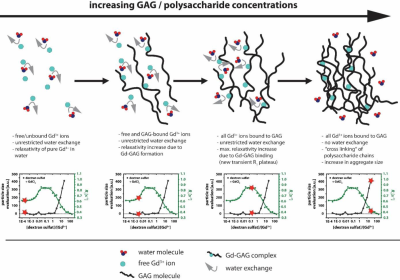
Glycosaminoglycans are heterogeneously distributed in the human body and create characteristic microenvironments with local hotspots. Studies showed that the binding of Gd-ions to polysaccharides leads to increased R1 values, which could explain the observed hyperintensities in biological tissues. Compared to free Gd-ions in solution, Gd-polysaccharide complexes have a decreased tumbling rate, which contributes to the higher relaxivity values [1,5,6]. The increased R1 values at low [GAG]/[GdCl3] ratios are over-compensated by a loss of R1, i.e. reduced relaxivity at higher polysaccharide concentrations. The shielding of Gd-ions on the R1 of the bulk water and thus the masking of the relaxation-observable concentration of the heavy metal ions is presumably caused by an increased occupation of the coordination sides of the Gd-ions by, e.g., sulfate groups from the added polysaccharides. This is accompanied by a Gd-ion mediated cross-linking of several polysaccharide chains (Fig.2), which in turn leads to an increase in the particle size as observed by light scattering data. Eventually, the intrinsic R1 of water can be reached for higher sugar concentrations, resulting in complete masking of the Gd-ions. In the case of polysaccharide structures such as dextran sulfate, this effect occurs more rapidly at smaller molecular weights (5 kDa) and leads to earlier masking of the actual Gd-ion concentration. This effect can be explained due to steric properties of the sugar structures and thus the easier accessibility of all coordination sites in shorter sugar chains for binding of Gd-ions.Conclusion:
Our results show that the binding of Gd-ions to polysaccharides and the associated increase or decrease in R1 depend strongly on the molecular weight of the polysaccharides in addition to the concentration ratios. By using model structures like dextran sulfate, we showed that, in addition to hyperintense signals, a clear masking of the Gd-ions due to the polysaccharide concentration can be caused in relaxometry experiments. We were able to demonstrate this effect for all molecular weights and we could show that the signal gain and signal loss is characteristic for each individual molecular size. This shows that research in this area must be continued such that the problem of deposited Gd-ions in biological tissues is not underestimated in the future.Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Grant No. 372486779 (SFB 1340); 289347353 (GRK 2260) and Koselleck Grant No. 316693477 (SCHR 995/5–1). Support by the Dieter Morszeck Stiftung to L.S. is also gratefully acknowledged.References
1. Taupitz, M., Stolzenburg, N., Ebert, M., Schnorr, J., Hauptmann, R., Kratz, H., ... & Wagner, S. (2013). Gadolinium‐containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast media & molecular imaging, 8(2), 108-116.
2. Kanda, T., Ishii, K., Kawaguchi, H., Kitajima, K., & Takenaka, D. (2013). High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology, 270(3), 834-841.
3. Radbruch, A., Weberling, L. D., Kieslich, P. J., Eidel, O., Burth, S., Kickingereder, P., ... & Bendszus, M. (2015). Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology, 275(3), 783-791.
4. Gianolio, E., Gregorio, E. D., & Aime, S. (2019). Chemical Insights into the Issues of Gd Retention in the Brain and Other Tissues Upon the Administration of Gd‐Containing MRI Contrast Agents. European Journal of Inorganic Chemistry, 2019(2), 137-151.
5. Werner, P., Schuenke, P, Taupitz, M. & Schröder, L. (2022). Investigating the Role of Sulfate Groups for the Binding of Gd3+ Ions to Glycosaminoglycans with NMR Relaxometry. ChemMedChem, 17 (13), e202100764 (2022)
6. Werner, P., Taupitz, M., Schröder, L., & Schuenke, P. (2021). An NMR relaxometry approach for quantitative investigation of the transchelation of gadolinium ions from GBCAs to a competing macromolecular chelator. Scientific Reports, 11, 21731 (2021)
Figures